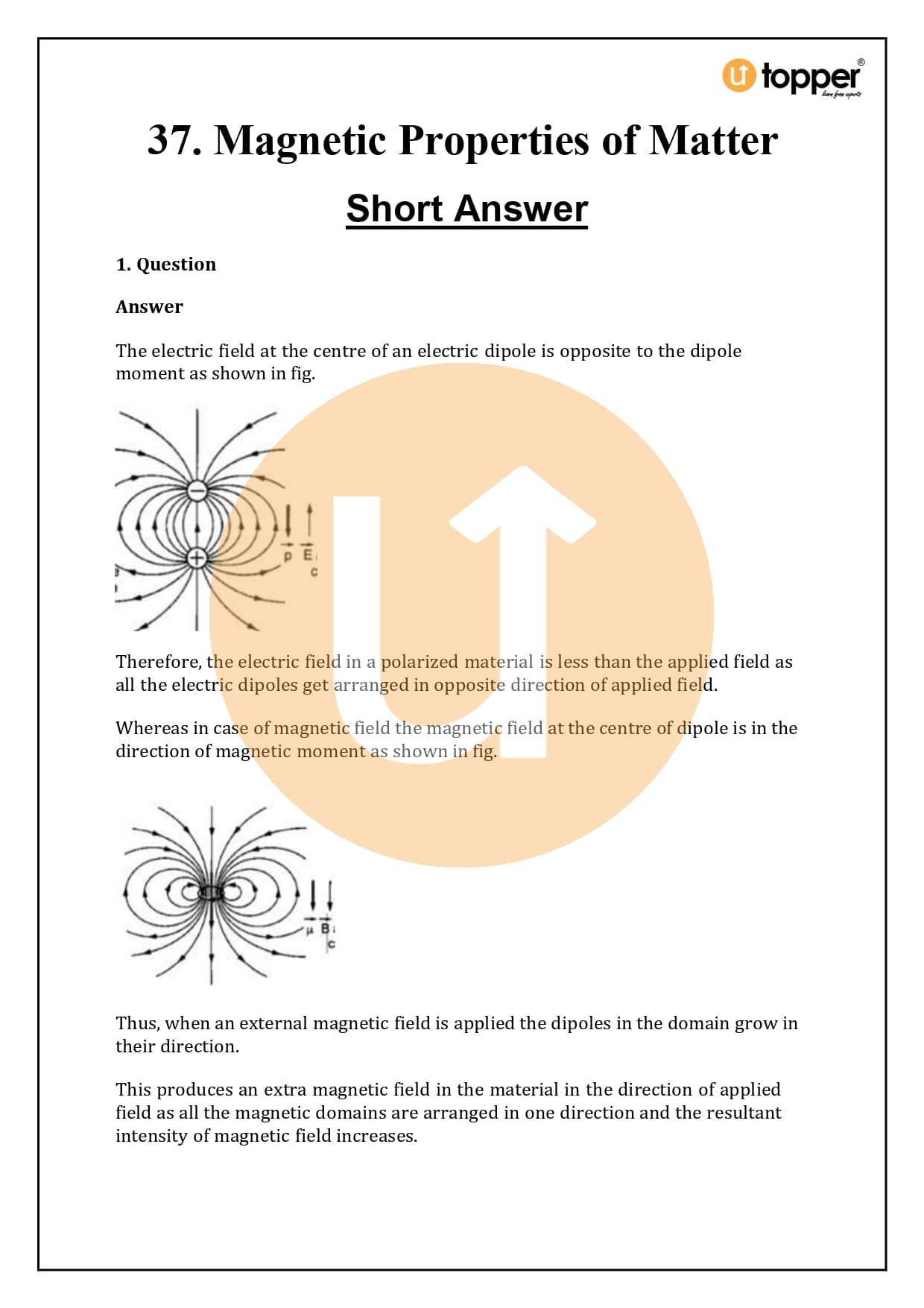
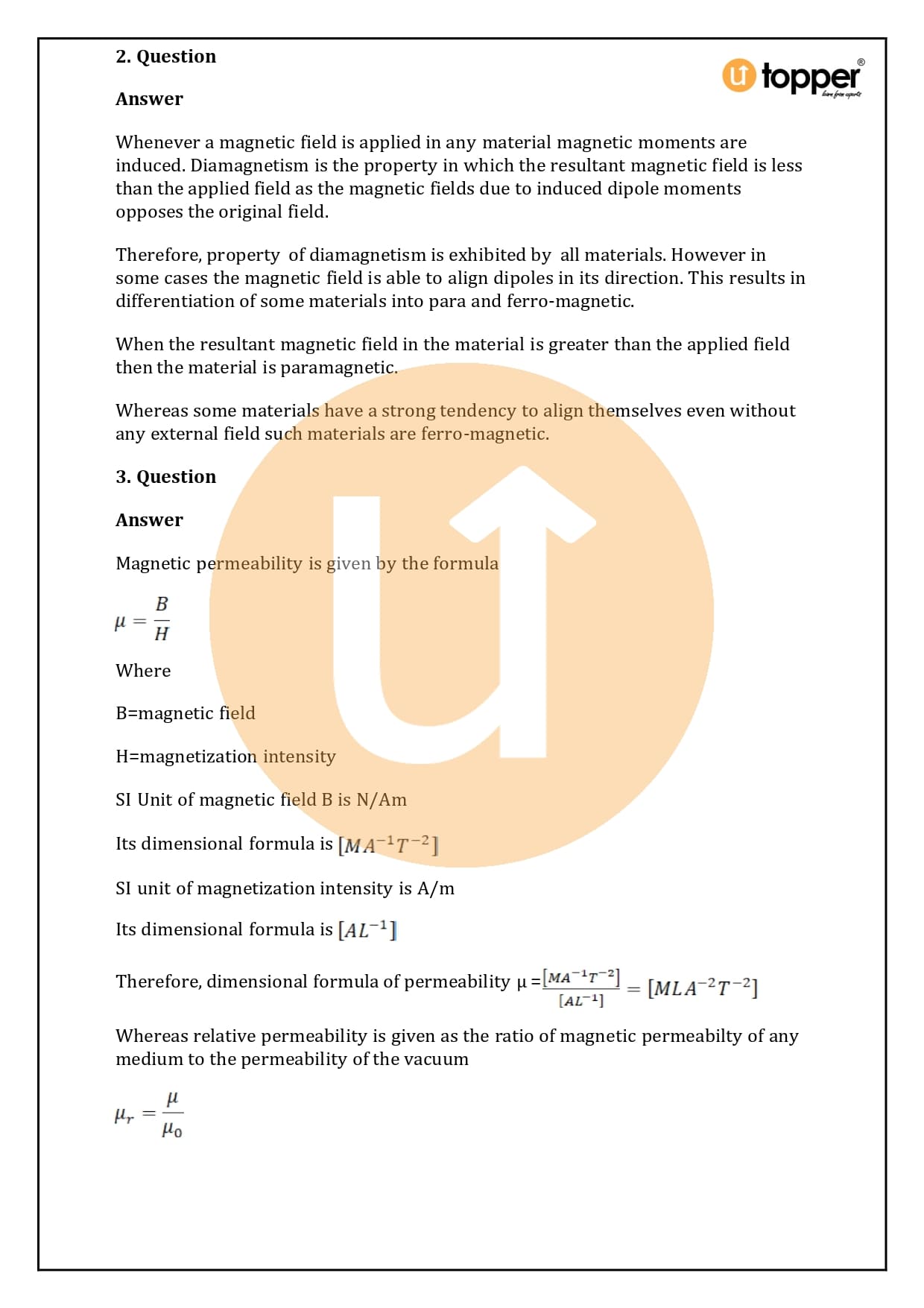
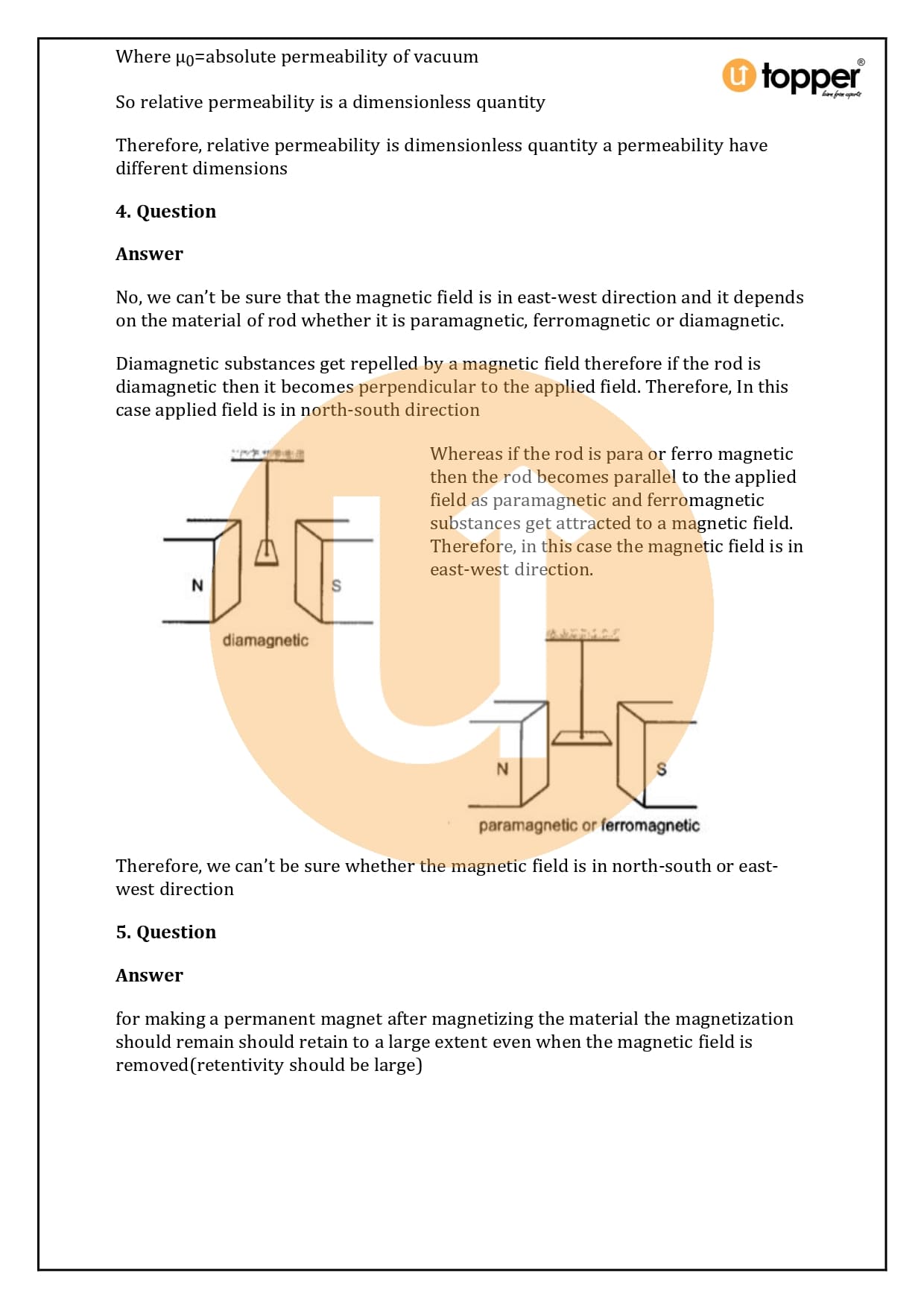
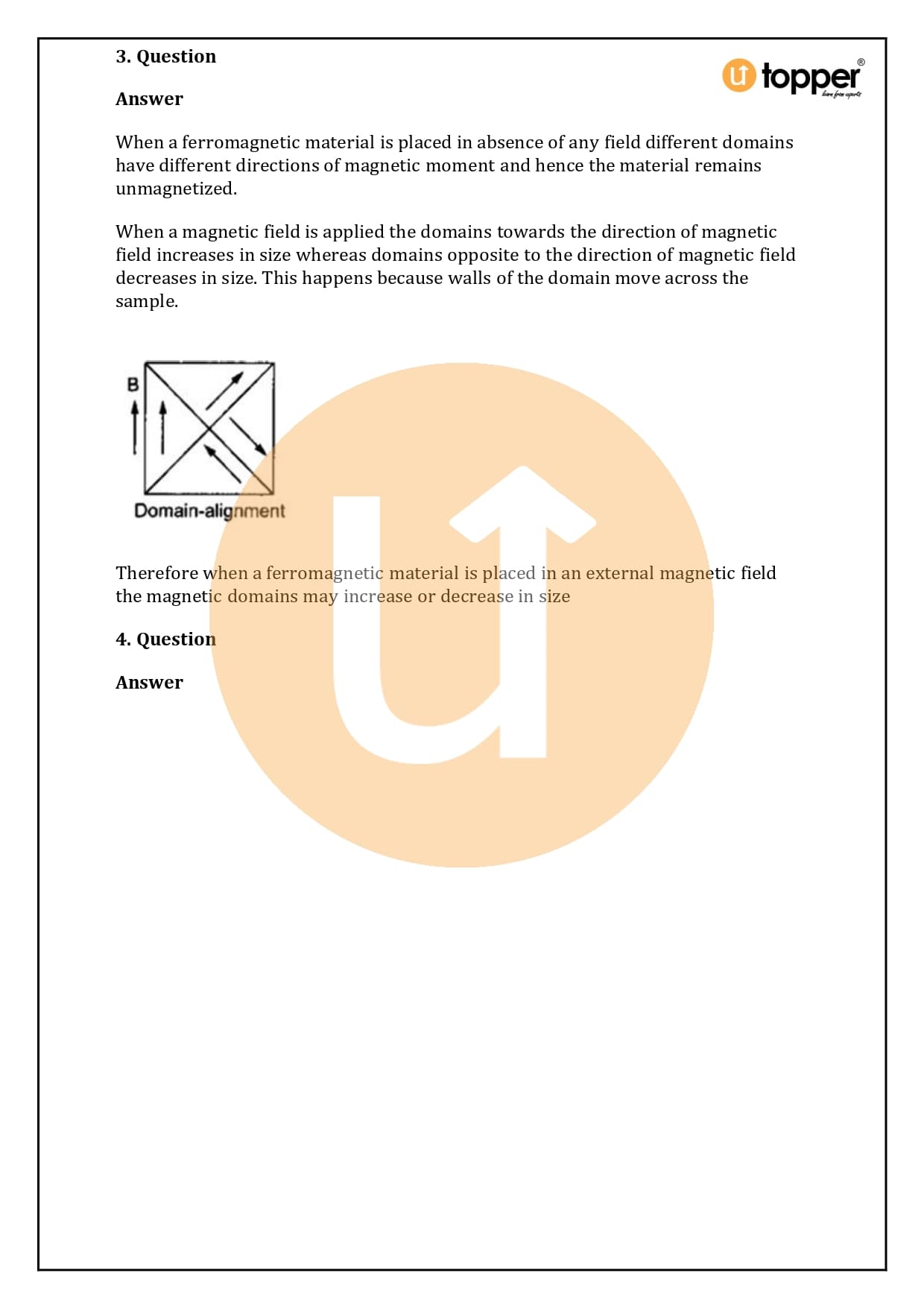
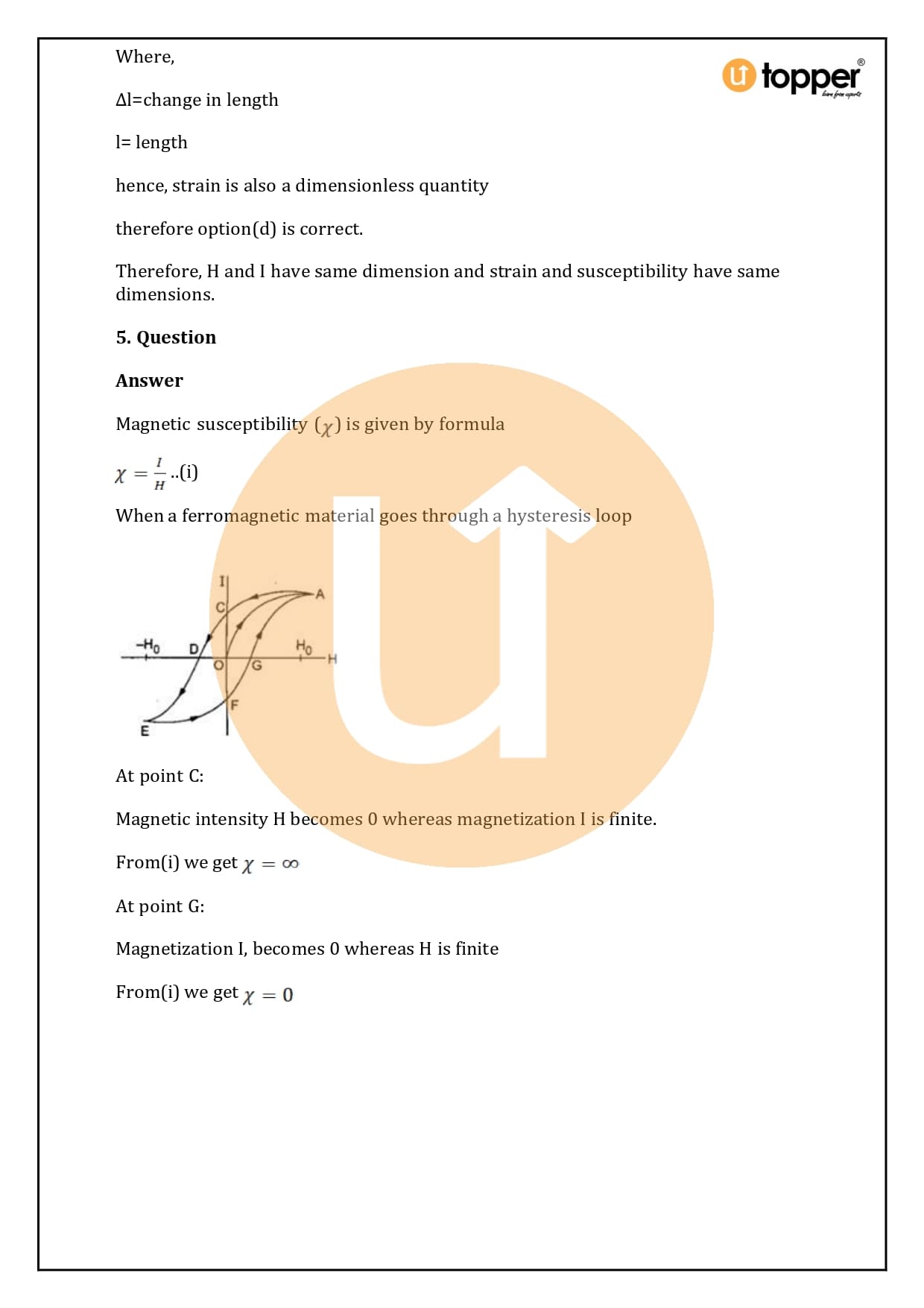
HC Verma Class 12 Physics Solutions Chapter 37
HC Verma Solutions of Concept of Physics Part 2 Chapter -37 Magnetic Properties of Matter
HC Verma Solutions for Vol 1 & 2 – Free PDF Download
HC Verma Class 12 Physics Solutions Chapter 37: Magnetic Properties of Matter PDF
This page has detailed, step-by-step explanations of every question in HC Verma Class 12 Physics Solutions Chapter 37. For example, in the chapter “Magnetic Properties of Matter” which is the important chapter of Volume 2 of HC Verma’s concept of physics for jee and neet, all of the questions are solved and the steps are explained to help you learn. Utopper is a smart way for students to go over the whole Physics Syllabus again and again. The questions and answers help them study in a way that will help them do well on their exams.
In the HC Verma Class 12 Physics Solutions Chapter 37 “Magnetic Properties of Matter” concept of physics, all of the questions are solved and the steps are explained to help you learn. On the Utopper website where students can get free Reference Book Solutions and other study materials like Revision notes, Sample papers, and Important Questions. Science will be easier to learn if you have access to HC Verma Solutions and solutions for other courses.
Click here to Buy Online – HC Verma Solutions Volume 2 Book
Here is a pdf of HC Verma Solutions Class 12 Volume 2 Chapter 37: Magnetic Properties of Matter















HC Verma Class 12 Physics Solutions
Chapter 23 – Heat and Temperature
Chapter 24 – Kinetic Theory of Gases
Chapter 25 – Calorimetry
Chapter 26 – Laws of Thermodynamics
Chapter 27 – Specific Heat Capacities of Gases
Chapter 28 – Heat Transfer
Chapter 29 – Electric Field and Potential
Chapter 30 – Gauss’s Law
Chapter 31 – Capacitors
Chapter 32 – Electric Current in Conductors
Chapter 33 – Thermal and Chemical Effects of Current
Chapter 34 – Magnetic Field
Chapter 35 – Magnetic Field due to a Current
Chapter 36 – Permanent Magnets
Chapter 37 – Magnetic Properties of Matter
Chapter 38 – Electromagnetic Induction
Chapter 39 – Alternating Current
Chapter 40 – Electromagnetic Waves
Chapter 41 – Electric Current through Gases
Chapter 42 – Photoelectric Effect and Wave-Particle Duality
Chapter 43 – Bohr’s Model and Physics of Atom
Chapter 44 – X-rays
Chapter 45 – Semiconductors and Semiconductor Devices
Chapter 46 – The Nucleus
Chapter 47 – The Special Theory of Relativity

About the chapter: HC Verma Solutions Class 12 Physics Chapter 37
Magnetism is a physical phenomenon, and one part of it is how magnetic forces work. Magnetism is the force that is put out by magnets when they attract or push away from each other. Basic particles’ magnetic moments and electric currents make a magnetic field, which acts on other magnetic and electric moments. How magnetic material depends on its pressure, temperature, and the magnetic field that is put on it. When these things change, a substance can show different kinds of magnetism.
There are different types and amounts of insulators and conductors on Earth, and all of them have magnetic properties.
In the 1800s, Michael Faraday was the first statistician to be found putting things into groups based on how magnetic they were. The strength of a magnetic field always decreases as you move away from it, but the math between strength and distance can be different
Four important things to know about how magnets work:
- A magnet has two ends, called poles. One is called the north pole or north-seeking pole, and the other is called the south pole or south-seeking pole.
- The south pole of the first magnet pulls toward the north pole of the second magnet, while the north pole of the first magnet pushes against the north pole of the second magnet.
- A magnet makes a magnetic field and is a sphere of magnetism that you can’t see or touch.
- A magnet’s north pole is roughly in the same direction as the Earth’s north pole, and vice versa. Because the Earth is made of magnetic materials and acts like a huge magnet.
Source of Magnetism
Magnetism comes from two sources:
- Electric current.
- Spinning magnetic moments of elementary particles.
Most of the magnetic properties of matter come from the magnetic moments of the atoms whose electrons are moving around them. The magnetic moments of atoms’ nuclei are much smaller than those of electrons, so they don’t make a difference in how magnetized material is.
Magnetic Properties of Matter
The matter has several magnetic properties, such as Magnetization, Diamagnetism, and Paramagnetism.
Magnetization
We will learn about magnetism and the idea of magnetic intensity in this section.
Magnetization, which is also called magnetic polarisation, is a vector field in electromagnetism that helps measure the number of induced or permanent magnetic dipole moments in a magnetic material. Magnetization is what William Gilbert found out about when he looked into the magnetism of matter. The direction of the change in this branch tells us whether the change is Axial or Diametric. We know that magnetism comes from the movement or spin of electrons in atoms or in nuclei. Magnetization comes from magnetism.
The theory of magnetism helps us put things into groups based on how magnetic they are. The response of a material to an outside magnetic field is what gives it its net magnetization. The net magnetic moment per unit volume of a sample material M is a measure of how magnetic that material is. The math formula for the magnetization field also called the M-field, is,
- M = mnetV
When put in a magnetic field, paramagnetic materials get a weak magnetization that goes away when the field is taken away. On the other hand, ferromagnetic and ferrimagnetic materials get a strong magnetization. That can be magnetized so that it stays magnetized even when there is no outside field. This makes a permanent magnet.
Diamagnetism
In September 1845, Michael Faraday found out about diamagnetism. This is a weak form of magnetism that is set up by a magnetic field from the outside. This makes a very small magnetic moment that goes in the opposite direction of the applied field. Diamagnetic is a type of magnetism in which a magnetic field pushes things away from each other. When a magnetic field is applied to a material, it creates an induced magnetic field in the material that is usually in the opposite direction. A magnetic field also pulls in materials that are paramagnetic or ferromagnetic.
When put inside a strong magnetic field, diamagnetic and electromagnetic materials move toward areas where the magnetic field is weak. In ferromagnetic and paramagnetic materials, the weak diamagnetic force is controlled by the magnetic dipoles’ ability to attract each other.
Michael Faraday was the first person to find out about diamagnetism. He did this in 1845. Also, Anton Brugmans noticed in 1778 that magnetic fields made bismuth move away from them. In chemistry, a simple rule of thumb is used to figure out if a particle, atom, or piece of iron is paramagnetic or diamagnetic. All of the electrons in a diamagnetic atom are paired up, and so is the material made from this atom. A paramagnetic material has an electron that doesn’t have a pair.
Paramagnetism
In a material with paramagnetism, there is an electron that doesn’t have a partner, so most of the atomic orbitals aren’t completely filled. So, this kind of atom is called a paramagnet. Paramagnetism is a type of magnetism in which a strong magnetic field weakly pulls on several different materials. Also, paramagnetism makes a magnetic field in the same direction as the magnetic field that is applied. In 1845, a British scientist named Michael Faraday found out about something called “paramagnetism.”
The arrangement of materials in paramagnetism is called “paramagnetic.” So, true paramagnets are set up according to the Curie-Weiss laws of magnetic susceptibility and show paramagnetism over a wide range of temperatures.
Curie’s law is important for this kind of magnetization. Curie’s law says that the magnetic susceptibility of paramagnetic materials goes down as their temperature goes up. It is shown to be;
- M = χH = C/T x H
- Where, M = magnetization,
- χ = susceptibility to magnetic fields,
- C = material-specific Curie constant,
- T = absolute (Kelvin) temperature,
- H stands for “extra magnetic field.”
Aluminum, oxygen, titanium, and iron oxide are all examples of materials that are not magnetic (FeO). Also, in chemistry, a simple rule of thumb is used to figure out if a particle, atom, or molecule is paramagnetic or diamagnetic. This rule depends on whether or not the electron is paired.
HC Verma Solutions Class 12 Physics Part 2 Complete Syllabus
- Chapter 23 – Heat and Temperature
- Chapter 24 – Kinetic Theory of Gases
- Chapter 25 – Calorimetry
- Chapter 26 – Laws of Thermodynamics
- Chapter 27 – Specific Heat Capacities of Gases
- Chapter 28 – Heat Transfer
- Chapter 29 – Electric Field and Potential
- Chapter 30 – Gauss’s Law
- Chapter 31 – Capacitors
- Chapter 32 – Electric Current in Conductors
- Chapter 33 – Thermal and Chemical Effects of Current
- Chapter 34 – Magnetic Field
- Chapter 35 – Magnetic Field due to a Current
- Chapter 36 – Permanent Magnets
- Chapter 37 – Magnetic Properties of Matter
- Chapter 38 – Electromagnetic Induction
- Chapter 39 – Alternating Current
- Chapter 40 – Electromagnetic Waves
- Chapter 41 – Electric Current through Gases
- Chapter 42 – Photoelectric Effect and Wave-Particle Duality
- Chapter 43 – Bohr’s Model and Physics of Atom
- Chapter 44 – X-rays
- Chapter 45 – Semiconductors and Semiconductor Devices
- Chapter 46 – The Nucleus
- Chapter 47 – The Special Theory of Relativity
Features of Utopper HC Verma Solutions Class 12 Physics Chapter 37
- Students can solve similar problems on their own with the help of HC Verma’s answers in the Utopper.
- Students are given answers that are correct and easy to understand.
- The solutions are given to match the level of understanding of a student in that class.
- The HC Verma solutions that Utopper gives the answer to and explains all of the questions in each chapter.
FAQ ( Frequently Asked Questions )
1. Where can I get HC Verma solutions?
Ans – Utopper offers HC Verma answers that are correct and have been worked out by experts. Our website, utopper.com, is where you can find HC Verma solutions. This is free, and if you study these answers, you can pass the JEE.
2. How Should I Study HC Verma For IIT JEE Preparation?
Ans – Students have used this book to study for JEE for the past twenty years, and they have all passed JEE. So, this book can’t be ignored. Once you’ve figured out how to solve the example problems, you can compare your answers to those in the book. If your answer is right, compare it to the solution to make sure you used the right steps to get there. If your answer is wrong, look at the other solutions to figure out “why” your answer is wrong. If you made a math or procedure mistake, you’ll need to find a way to fix it if you want to do well on an exam like the JEE.
3. Is HC Verma enough for the JEE Advanced?
Ans – Is the H.C. Verma book enough to prepare for the JEE Main and Advanced? H.C. Verma is a good book for getting ready for the JEE, and it goes into detail about each topic.
4. Who is better: SL Arora or HC Verma?
Ans – If you want to take the boards, SL Arora will do fine, and you won’t have to take HCV. But if you are studying for competitive exams like IIT/JEE mains or advanced, you should definitely go for HCV because it has more advanced problems and will help you solve more complicated problems.
5. What is the best way to solve HC Verma?
Ans- Start from the beginning. Don’t skip around or move in a random way. Before you try to solve the problems, read the whole chapter. Take your time reading and make sure you understand the ideas.
6. Is only HC Verma Solutions enough for NEET Preparation?
Ans – HC Verma won’t help you enough on its own. It will help you with one subject, but you’ll need to look at other books for the rest. You can also use NCERT books, which are very helpful for passing the NEET exam. For the NEET exam, you need a lot of practice, which you can get from reading about many different things.
