Introduction to Amines – Compounds Containing Nitrogen
Amines are one of the most important groups of organic compounds formed when one or more hydrogen atoms of an ammonia molecule are replaced with an alkyl group.
You can Read More Chemistry Articles.
What are Amines?
In general, an amine is a functional group containing a nitrogen atom with a lone pair. Structurally, amines resemble ammonia because nitrogen may form bonds with up to three hydrogen atoms. Additionally, it possesses varied properties based on carbon connection.
Compounds containing nitrogen bonded to a carbonyl group are known as amides; their structure is R–CO–NR′R′′ and their characteristics differ from those of amines.

Amines are chemical molecules that contain lone pairs of nitrogen atoms. In essence, they are generated from ammonia (NH3) in which one or more hydrogen atoms are substituted by an alkyl or aryl group, hence their respective names of alkylamines and arylamines.
Amine Structure
Nitrogen has five valence electrons and is hence a trivalent element with a lone pair. According to the VSEPR theory, the nitrogen in amines is sp3 hybridized and, due to the existence of a lone pair, has a pyramidal structure rather than the tetrahedral form typical of most sp3 hybridized molecules. Depending on the configuration of amines, each of the three sp3 hybridized orbitals of nitrogen overlap with orbitals of hydrogen or carbon. The C-N-H angle in amines is less than 109 degrees, which is a hallmark angle of tetrahedral geometry, due to the existence of a lone pair. The amines’ angle is around 107 degrees.
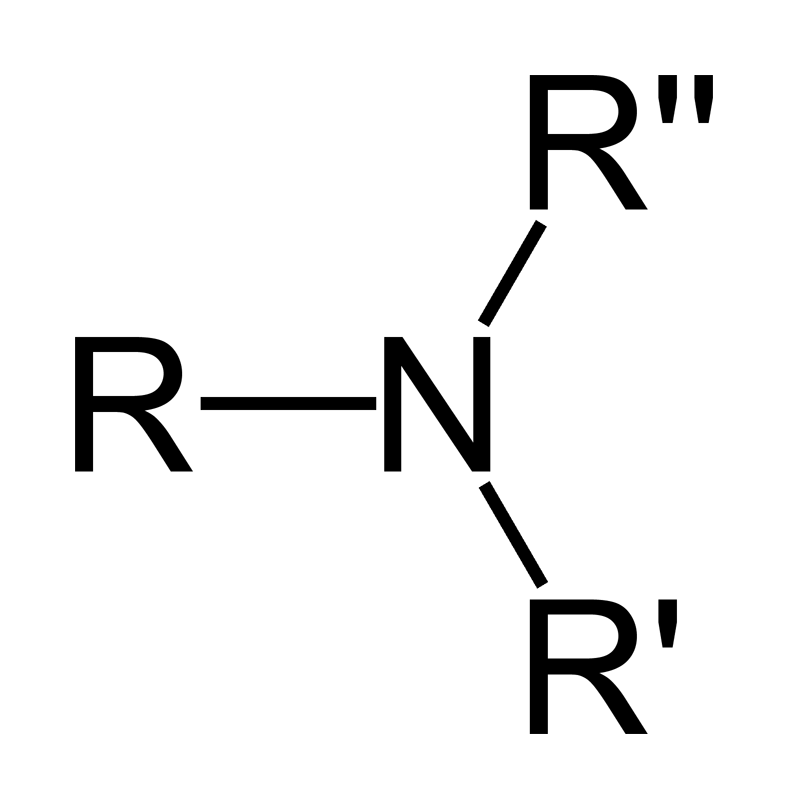
Occurrence of Amines
Amines occur naturally in proteins, vitamins, hormones, etc., and they are also synthesized to produce polymers, medicines, and pigments.
Types of Amines
On the basis of the manner in which hydrogen atoms are replaced by an ammonia molecule, amines can be categorized into four distinct categories.

Primary Amines
When an alkyl or aryl group is substituted for one of the hydrogen atoms in the ammonia molecule.
Example: Methylamine (CH3NH2) and Aniline (C6H5NH2)
Secondary Amines
Two organic substituents replace the ammonia molecule’s hydrogen atoms to make an amine.
Dimethylamine (CH3)2NH and Diphenylamine (C6H5)2NH are examples.
Tertiary Amines
When all three hydrogen atoms are replaced with an organic substituent, the resulting group may be aryl or aromatic.
Trimethylamine N(CH3)3, Ethylenediaminetetraacetic acid are examples of compounds (EDTA)
Cyclic Amines
These amines have an aromatic ring structure and are secondary or tertiary.
Eg: Piperidine (CH2)5NH, Aziridines C2H5N
Preparation of Primary Amines
1. Making of amines from halogenoalkanes
This procedure will be conducted in an airtight tube. Here, haloalkanes will be heated with a concentrated ammonia-ethanol solution. The mixture cannot be heated under reflux because ammonia would escape the container as a gas.

Regarding the preparation of primary amine from halogenoalkane, there are two steps to the reaction. In the initial stage, salt is produced. Here, the salt is ethyl ammonium bromide. It is similar to ammonium bromide except that one of the ammonium atom’s hydrogens has been replaced by an ethyl group.

The reaction between ammonia and salt is reversible. It is demonstrated by the preceding reaction.
2. Reduction of nitriles
By reducing nitriles using lithium aluminium hydride, primary amines can be produced. This procedure is mostly used to create amines with one additional carbon atom than the beginning amine.
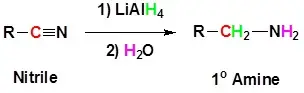
3. Gabriel phthalimide synthesis
Through Gabriel synthesis, it is simple to acquire primary amines. In this procedure, phthalimide is treated with ethanolic potassium hydroxide to produce phthalimide potassium salts. This produces primary amine upon additional heating with alkyl halide followed by alkaline hydrolysis. Because aryl halides do not undergo nucleophilic substitution with the anion generated by phthalimide, it is not possible to prepare aromatic primary amines.

Basicity of Amines
Similar to ammonia, primary and secondary amines contain protic hydrogens and, as a result, exhibit some acidity. Tertiary amines, on the other hand, lack protic hydrogen and so lack acidity.
The pKa value of primary and secondary amines is approximately 38, making them extremely weak acids. In contrast, the pKb value is around 4. This renders amines far more basic than acidic. Thus, an amine’s aqueous solution is highly alkaline.
Uses of Amines
Amines have numerous applications in everyday life. Listed below are some uses for amines:
- It is utilized in water purification, drug production, and the creation of insecticides and herbicides.
- It contributes to the synthesis of amino acids, the building blocks of proteins in living organisms. Several types of vitamins are also produced by amines.
- Serotonin is an essential amine that serves as one of the principal neurotransmitters. It governs the sensations of hunger and is essential for the normal operation of the brain.
- Analgesic medications such as Morphine and Demerol, generally known as analgesics, are derived from amines.
Students should now have a basic understanding of the fundamental ideas of amines, but if they want to learn more about the chemical and physical features of amines, Visit Utopper.com
Frequently Asked Questions – FAQs
Q.1 What is the basicity order of Amines?
Because amine has lone pair electrons on its nitrogen atom, it can contribute a pair of electrons to make a base. The basicity of amines is determined by stectric factors, the electronic factor, and the solvent factor.
When R=Me, the order of basicity of amine in the gaseous state is 30 > 20 > 10
When R= Me, the order of basicity of amine in aqueous solution is 20 > 10 > 30.
When R = Et, the order of basicity of amine in aqueous solution is 20 > 30 > 10
Q.2 What is the difference between amine and amide?
Amines and amides are two distinct chemical compound kinds. The distinction between amine and amide is the existence of a carbonyl group in their structures; amines do not contain carbonyl groups connected to the nitrogen atom; the formula for amine is RNH2, whereas the formula for amide is R-CO- NH2.
Q.3 What are the uses of amines ?
Amines are utilized for water purification, drug production, and the creation of insecticides and pesticides. It also contributes to the synthesis of amino acids, the building blocks of proteins.
Q.4 Are amines harmful?
In general, the majority of aliphatic amines contained in food are involved in the synthesis of amino acids, the fundamental building blocks of proteins.
Some aromatic amines may be poisonous in nature and produce skin irritants, however these are not very toxic in nature.
Q.5 How many types of amine are there?
Aliphatic or aromatic amines are classed as primary, secondary, or tertiary according to the number of alkyl or aryl groups connected to the nitrogen atom.

