What is Ammonium persulfate?
Ammonium persulfate is the scientific name for (NH4)2S2O8, an inorganic compound. It is also known as Ammonium peroxydisulfate and Diammonium persulfate. It has numerous applications in polymer chemistry, including bleaching, cleaning, and etching.
You can Read More Chemistry Articles.
Diammonium peroxydisulfate is a white substance that is crystalline and odorless. It is easily soluble in water and a powerful oxidizer.
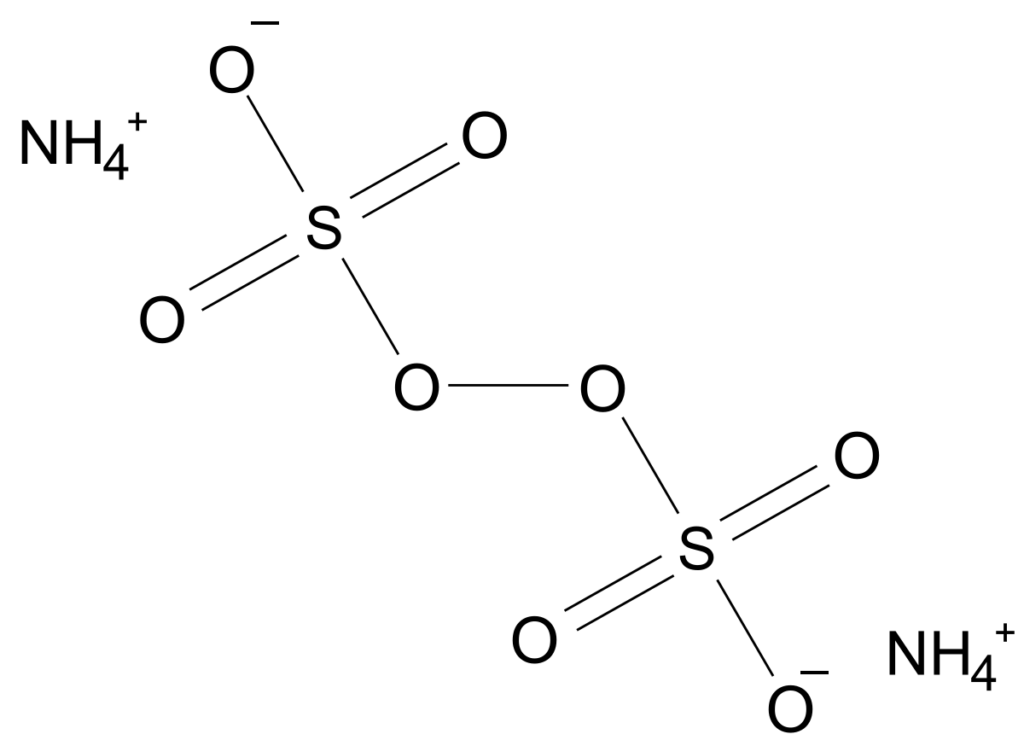
Ammonium persulfate structure – (NH4)2S2O8
Chemical Properties of Ammonium persulfate – (NH4)2S2O8
| (NH4)2S2O8 | Ammonium persulfate |
| The molecular weight of (NH4)2S2O8 | 228.18 g/mol |
| The density of Ammonium persulfate | 1.98 g/cm3 |
| The flash point of Ammonium persulfate | Non-flammable |
| Melting Point of Ammonium persulfate | 120 °C |
Production of Ammonium persulfate
Electrolysis of a cold concentrated solution of ammonium bisulfate or ammonium sulfate in sulfuric acid (H2SO4) at a high concentration produces ammonium peroxydisulfate. Hugh Marshall initially described this technique.
Health hazards
Diammonium persulfate is mildly harmful when inhaled. Dust irritates the eyes and causes rashes on the skin.
When this substance is heated, harmful sulfuric acid oxides and nitrogen oxides may be released. It is an inflammable chemical that accelerates the combustion of other compounds. In a fire, it produces annoying poisonous vapors. Extremely explosive when in contact with reducing agents or flammable materials.
Uses of Ammonium persulfate
- Ammonium persulfate is used as an adjuvant in hydrogen peroxide bleaching.
- Applied to printed circuit boards.
- Used as an initiator in the polymerization of olefins.
- Used in for photography.
- Used as a food preservative agent.
- Used in as an oxidant.
- Used in to clean contaminated yeast.
- Used for eliminating pyrogallol stains.
- Used as a battery depolarizer.
- Used as a common hair bleaching agent.
Learn about the Structure, physical and chemical properties of another chemical compound at Utopper.com.
Frequently Asked Questions – FAQs
Q.1 What is ammonium persulfate used for?
Ammonium persulfate is utilized as a bleaching agent, as an initiator for olefin polymerization, in photography, hair bleach, and in batteries. Infected yeast, food preservatives, and printed circuit boards are also cleaned with ammonium persulfate.
Q.2 How do you get 10% ammonium persulfate?
By dissolving 1 gram of ammonium persulphate in 10 milliliters of water, we can obtain 10% ammonium persulphate.
Q.3 Where do you store ammonium persulfate?
Ammonium persulphate is stored on dry ice at ambient temperature (15-30 °C).
Q.4 Why is ammonium persulfate used in SDS?
When preparing sodium dodecyl sulphate polyacrylamide gel electrophoresis, ammonium persulfate is used with TEMED (Tetramethylethylenediamine) as a catalyst for polymerization of acrylamide (SDS PAGE).
Q.5 Is ammonium persulfate toxic?
Ammonium persulfates are mildly harmful when inhaled. Dust irritates the eyes and creates rashes on the skin. Higher doses may result in pulmonary edema (fluid accumulation in the lungs), a medical emergency.

