What is Aluminium Sulphate (Al2(SO4)3)?
Aluminium sulfate is a chemical compound with the formula (Al2(SO4)3).
Aluminium sulphate is also known as Dialuminum trisulphate and Filter Alum. In its anhydrous form, it is a white crystalline solid, however, in its solution form, it appears as a colourless liquid. Each type is non-toxic and non-combustible.
Aluminium sulphate is water-soluble but ethanol-insoluble. It is odourless and has a sweet, moderately astringent flavour. It emits poisonous gases of sulphur oxides upon decomposition. Aluminium is corroded by the solution of aluminium sulphate. This chemical is created in the laboratory by combining sulphuric acid with aluminium hydroxide.
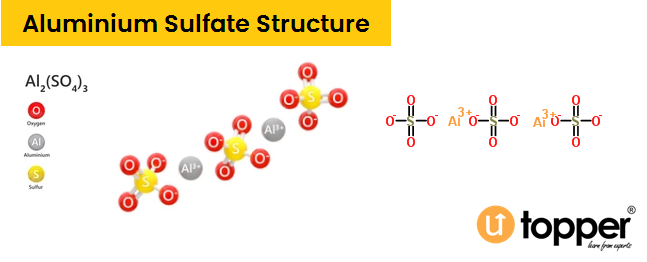
Aluminium Sulphate Structure
Properties of Aluminium Sulphate – Al2(SO4)3
| Al2(SO4)3 | Aluminium sulphate |
| Molecular Weight/ Molar Mass | 342.15 g/mol |
| Density | 2.672 g/cm3 |
| Boiling Point | 214° F |
| Melting Point | 770 °C |
Preparation of Aluminium Sulphate
Aluminium sulphate is produced by reacting freshly precipitated aluminium hydroxide with a suitable amount of sulfuric acid. The resulting solution is evaporated and allowed to crystallize. Pure, glossy crystals, granules, or powder are available.
2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6 H2O
Heating aluminium metal in a solution of sulfuric acid can also make aluminium sulphate.
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2↑
Aluminium Sulphate (Al2(SO4)3 ) Uses
- It’s a component of baking soda.
- It is used in gardening to balance the pH of the soil.
- Used in water filtration.
- It is used in the dying of fabric.
- It is used to manufacture paper.
- Used for printing on fabric.
- It is used as an accelerator and waterproofing ingredient in concrete.
- It is a component of firefighting foam.
- It is used in the treatment of sewage.
- It serves as a fire retardant.
Frequently Asked Questions – FAQs
Q.1 How is aluminium sulphate made?
Aluminum sulphate is produced by reacting freshly precipitated aluminum hydroxide with the suitable amount of sulfuric acid. The resulting solution is evaporated and allowed to crystallize. Pure, glossy crystals, granules, or powder are available.
Q.2 What type of compound is aluminium sulphate?
Aluminium sulphate is an ionic chemical, composed of positive and negative ions. Ions are charged atoms, which may be monatomic ions (single atoms) or polyatomic ions (many atoms) (multiple atoms combined to form a charged part). Al+3 is the polyatomic ion of aluminium, while (SO4)-2 is the polyatomic ion of sulphate.
Q.3 How dangerous is aluminium sulphate?
Aluminium sulphate is an irritant to the skin and eyes, hence gloves and eye protection should be worn when handling it. When aluminium sulphate is ingested, it can transform into a highly corrosive sulphuric acid, posing a slight health risk.
Q.4 Why is aluminium sulphate soluble in water?
Al2(SO4)3 is a chemical compound that produces aluminium sulphate. It is soluble in water and is largely employed as a coagulating agent (increasing particle collision by neutralizing charge) in drinking water and wastewater treatment plants as well as in paper manufacturing.
Q.5 What is the smell of Aluminium sulphate?
Aluminium sulphate is an odorless, white, crystalline, mildly water-soluble, and organic solvent-insoluble chemical. This is acidic in flavor.
Experts at Utopper.com explain the structure, physical and chemical properties of Aluminium sulphate (Al2(SO4)3).

