What is Ionization?
Ionization of a compound is the splitting of a neutral molecule into charged ions when it comes into contact with a solution. When a compound is dissolved in a solvent, it breaks apart into positive and negative ions called electrolytes. The degree of ionization is the ratio of the number of molecules that are breaking apart to the total number of molecules.
𝞪 = number of molecules undergoing dissociation/ total number of molecules
Where 𝞪 is called the degree of ionization
Arrhenius’s concept of Acid and base ionization
Arrhenius’s theory says that acids are compounds that break apart in water to release hydrogen ions (H+) (aq). On the other hand, bases are substances that give off hydroxyl ions (OH–(aq)) in water.
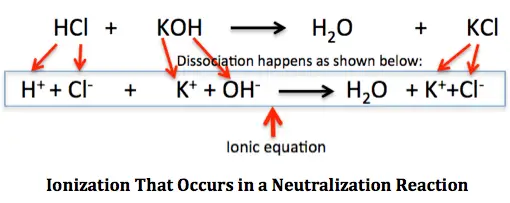
Since most ionization happens in water, Arrhenius’s theory is a key part of explaining how acids and bases become charged. We can figure out how strong acids and bases are by looking at how much ionization they have. Different acidic and basic compounds have different amounts of ionization. Some acids, like perchloric acid (HClO4) and hydrochloric acid (HCl), completely break apart into their individual ions in water. The name for these acids is “strong acids.”
When acids are ionized, they give off hydrogen ions. This means that acids are proton donors. In the same way, some bases, such as lithium hydroxide (LiOH) and sodium hydroxide (NaOH), completely separate into their ions in water. We call these “strong bases.” When these bases are broken down, they give off hydroxyl ions (OH–).
So, the amount of ionization of acids and bases depends on how much compounds break apart into their individual ions. When compared to weak acids and bases, strong acids and bases have a lot of ions. A strong acid also means that it is a good proton donor, while a strong base means that it is a good proton acceptor. For example, the weak acid HA can split apart.
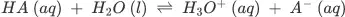
Explanation of Arrhenius acid and base ionization
This reaction shows that the acid dissociation equilibrium is dynamic and that proton transfer can happen both forward and backward. If HA is more likely than H3O+ to give up a proton, then HA acts as a strong acid compared to H3O+. Since the acid that is stronger gives a proton to the base that is stronger. The equilibrium moves in the direction of making an acid and a base that are both weaker. Most of the time, the conjugate bases of strong acids are weaker than the conjugate acids of strong bases. This is because strong acids and bases have a lot of ions in them.
Determination of ionization constant of acid and base
Based on what we’ve talked about so far, we can say that when an Arrhenius acid splits, it does so in the following way.
HA (aq) + H2O (l) ⇌ H3O+(aq) + A−(aq)
So according to the equilibrium constant;
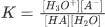
K=[H3O+][A−][HA][H2O]
K [H2O] = [H3O+] [A−] / [HA]
Ka = [H3O+] [A−] / [HA]
Where Ka is known as the ionization constant of acid, for stronger acid Ka value will be higher.
Similarly for Arrhenius’s base
BOH (aq) + H2O (l) ⇌ B+ (aq) + OH−(aq)
So according to the equilibrium constant;
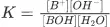
K=[B+][OH−][BOH][H2O]
K [H2O] = [B+] [OH−] / [BOH]
Kb = [B+] [OH−] / [BOH]
Where Kb is known as the ionization constant of the base, for a stronger base, the Kb value will be higher.
Examples of Acid and Base ionization
The equation for the ionization of CH3COOH and NH4OH.
Ionization of acetic acid in aqueous solution: CH3COOH + H2O ⇋ H3O+ + CH3COO–
Ionization of ammonium hydroxide in aqueous solution; NH4OH + H2O ⇋ NH4+ + OH–
Frequently Asked Questions – FAQs
Q.1 What happens when acids and bases are ionized?
Arrhenius’s theory says that acids are the electrolytes that ionize in water to form hydrogen ions (H+) (aq). On the other hand, bases are the electrolytes that give off hydroxyl ions, OH(aq), in an aqueous medium.
Q.2 How do you know if something ionizes completely?
With the value of the degree of ionization, we can tell if the compound is fully ionized or not. The ratio of the number of molecules that are breaking apart to the total number of molecules is the degree of ionization. If the value of the degree of dissociation is 1, it means that the molecule has lost all of its electrons.
Q.3 Is HCl a weak or strong acid?
HCl is a strong acid because it breaks apart almost completely and has a high ionization value.
Q.4 What is the percent ionization formula?
The percentage of ionization of a weak acid or base is equal to the ratio of the concentration of the ionized weak acid or base to the concentration of the acid or base at the start, multiplied by 100.
Q.5 What is the ionization of the base?
A base is an ionized substance that, when dissolved in water, makes hydroxide ions (OH–) and the acid that is its conjugate. Kb stands for the constant for the ionization of the base at the point of equilibrium. The Kb value of a strong base is higher than that of a weak base.
For detailed discussions on acids and bases and other topics of chemistry, log on to Utopper.com.

