What is Acid Rain?
Acid Rain is, as its name suggests, the precipitation of acid in the form of rain. Acid Rain occurs when air contaminants such as oxides of nitrogen and sulfur react with precipitation and fall with the rain.
Acid Rain Definition
Acid rain consists of water droplets that are extremely acidic due to air pollutants, notably the disproportionate levels of sulfur and nitrogen generated by vehicles and factories. It is sometimes referred to as acid rain because it encompasses numerous types of acidic precipitation.
There are two types of acidic deposition: wet and dry. Wet deposition refers to any precipitation that removes acids from the atmosphere and deposits them on the earth’s surface. Dry deposition of harmful particles and gases adheres to the ground through dust and smoke in the absence of precipitation.
Forms of Acid Deposition
Wet Deposition
We most usually consider acid rain to be a wet deposition. The sulfuric acid and nitric acids produced in the sky combine with precipitation, fog, or hail to reach the earth.
Dry Deposition
Acidic particles and gases can also deposit from the environment as dry deposition in the absence of moisture. The acidic particles and gases may swiftly deposit on surfaces (water, vegetation, structures) or react during atmospheric transit to generate bigger particles that are hazardous to human health. When the stored acids are washed off a surface by the next rain, acidic water flows over and through the ground, which can be harmful to plants and animals, such as insects and fish.
The quantity of atmospheric acidity that falls to the earth through dry deposition is dependent on the amount of precipitation a region receives. For example, in arid regions, the ratio of dry to wet deposition is greater than in regions that receive several inches of annual precipitation.
What Causes Acid Rain?
Sulfur and Nitrogen particles combine with the wet components of rain to produce acid rain. Sulfur and Nitrogen particles that combine with water can originate from either man-made sources, such as industrial emissions, or from natural causes, such as lightning strikes in the atmosphere that release nitrogen oxides and volcanic eruptions that release sulfur dioxide.
According to the Royal Society of Chemistry, which deems him the “father of acid rain,” Scottish chemist Robert Angus Smith coined the term “acid rain” in 1852. Smith selected the term while researching the chemistry of rainwater near industrial cities in England and Scotland.
Water and carbon dioxide combine to generate mild carbonic acid, which is not highly hazardous on its own. This is the case for the rain that falls on a regular basis, despite the fact that it is not pure. The reaction taking place is:
H2O (l) + CO2 (g) ⇌ H2CO3 (aq)
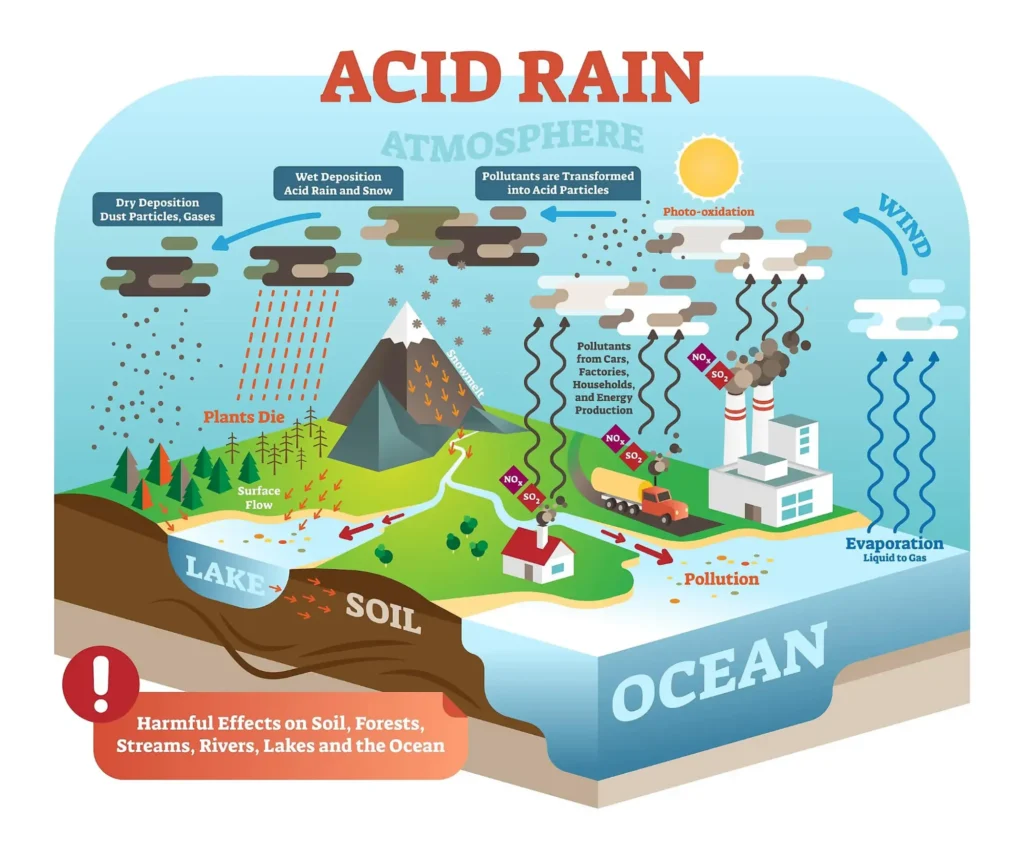
The pH of normal rainwater is approximately 5,7, giving it an acidic character. The wind carries away the oxides of nitrogen and sulfur along with the dust particles. They settle on the surface of the earth after falling as precipitation. Acid rain is a byproduct of human activities that produce nitrogen oxides and sulfur oxides into the atmosphere. Example: the use of fossil fuels and unethical waste disposal practices.
The oxidation of sulfur dioxide and nitrogen dioxide leads to the creation of sulphuric acid and nitric acid, respectively. The subsequent reaction clarifies the acid production process:
2SO2 (g) + O2 (g) + 2H2O (l) → 2H2SO4 (aq)
4NO2 (g) + O2 (g) + 2H2O (l) → 4HNO3 (aq)
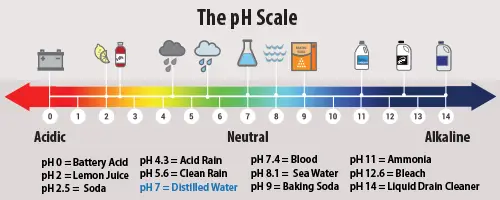
Measuring Acid Rain
The neutral point on the pH scale that measures acidity and alkalinity is 7. The lower the pH (less than 7) of a substance, the more acidic it is; the higher the pH (greater than 7) of a substance, the more alkaline it is. Normal rain has a pH of about 5.6; it is somewhat acidic due to the dissolution of carbon dioxide (CO2), which forms mild carbonic acid. Typically, acid rain has a pH between 4.2 and 4.4.
Effects of Acid Rain
- Acid rain is extremely damaging to agriculture, vegetation, and animals. It eliminates all nutrients essential to the growth and survival of plants. By altering the soil’s chemistry, acid rain is detrimental to agriculture.

- It causes respiratory problems in both people and animals.
- When acid rain falls and runs into rivers and ponds, the aquatic ecology is impacted. It causes water pollution by altering the chemical composition of the water to a form that is detrimental to the survival of aquatic ecosystems.
- Acid rain also causes water pipelines to corrode, resulting in the leaching of heavy metals such as iron, lead, and copper into drinking water.
- It causes harm to stone and metal structures and monuments.

Real-Life Examples
One of the seven wonders of the world, the Taj Mahal, is significantly damaged by acid rain. Numerous industries in the city of Agra produce sulfur and nitrogen oxides into the atmosphere. The continued use of low-quality coal and firewood as home fuels contributes to this issue. The following reaction occurs between acid rain and marble (calcium carbonate):
CaCO3(s) + H2SO4(l) → CaSO4(s) + H2O(l) + CO2(g)

This magnificent structure has corroded due to the production of calcium sulfate.
- The copper Statue of Liberty has been deteriorating for almost 30 years due to the cumulative effects of acid rain and oxidation and is now turning green.

Prevention of Acid Rain
- The only protection we can take against acid rain is to reduce our emissions of nitrogen and sulfur oxides.
- Acid rain is hazardous to animals, vegetation, and historical structures.
- Responsible citizens should be aware of the adverse consequences they cause and the industries that emit nitrogen and sulfur pollutants in an unethical manner.
Frequently Asked Questions – FAQs
Q.1 What is acid rain, and what causes it?
Acid rain is created by a chemical reaction that occurs when sulfur dioxide and nitrogen oxides are discharged into the atmosphere. These molecules can ascend very far into the sky, where they combine with water, oxygen, and other chemicals to generate acid rain, a type of acidic pollution.
Q.2 What are 3 effects of acid rain?
The biological effects of acid rain are most pronounced in marine ecosystems such as streams, lakes, and marshes, where toxic fish and other species thrive. As it passes through the soil and into streams and lakes, acidic precipitation can leach aluminum from soil clay particles.
Q.3 What will happen if we don’t stop acid rain?
Sulfur dioxide and nitrogen oxide are the two most prevalent components of acid rain. Since the acid is found in fruits, vegetables, and animals, it can potentially affect humans. In other words, if acid rain does not cease and we continue to consume these foods, we can become really ill. In general, acid rain has indirect effects on men.
Q.4 What is acid rain? What are its harmful effects?
It has been demonstrated that acid rain has negative effects on trees, freshwaters, and soils, destroys insects and aquatic life, causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and sculptures, in addition to having negative effects on human health.
Q.5 What are three ways to reduce acid rain?
Utilize alternative energy sources, such as solar and wind power. Renewable energy sources assist prevent acid rain since they produce significantly fewer emissions. There are additional sources of electricity, including nuclear power, hydropower, and geothermal energy. Nuclear and hydropower have the most extensive application among these technologies.
Q.6 How does acid rain affect plants?
Acid rain can negatively impact plant health. The pH of the soil where the plant is growing is altered by acid rain, influencing the plant’s overall growth. In addition, it binds or dissolves important soil minerals like nitrogen and phosphorus and transports them away.

