What is Acid Dissociation Constant (Ka)
Acid dissociation constant (also known as acidity constant or acid-ionization constant; abbreviated Ka) is a quantitative measure of an acid‘s strength in solution. It is a chemical reaction’s equilibrium constant.
HA ⇌ A– + H+
acid-base reactions are known as dissociation. HA is an acid that dissociates into A, its conjugate base, and H+, the hydrogen ion. When both forward and reverse processes are operating at the same rate, the concentrations of the system’s components will not change over time.
The constant of dissociation is defined by
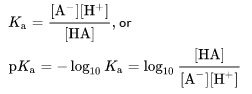
The amounts in square brackets reflect the equilibrium concentrations of species.
Theoretical background
The acid dissociation constant results directly from the underlying thermodynamics of the dissociation reaction; the pKa value is proportional to the standard Gibbs free energy change for the reaction.
The pKa value varies with temperature and can be qualitatively comprehended using Le Chatelier’s principle: when the reaction is endothermic, Ka rises and pKa falls with rising temperature; when the reaction is exothermic, Ka rises and pKa falls with increasing temperature.
Definitions
According to Arrhenius’s original molecular definition, an acid is a substance that dissociates in an aqueous solution, releasing the hydrogen ion H+ (a proton).
HA ⇌ A– + H+
H2O ⇌ H+ + OH–
The equilibrium constant for this dissociation reaction is the dissociation constant. Arrhenius proposed that the dissociation be described as an acid-base reaction because the released proton combines with a water molecule to generate the hydronium ion H3O+ (free protons do not exist in solution):
HA + H2O ⇌ A– + H3O+.
Bronsted and Lowry then generalized this to a proton exchange reaction:
Acid + Base ⇌ Conjugate base + Conjugate acid.
The acid loses a proton, leaving a conjugate base; the proton is transferred to the conjugate base, creating a conjugate acid.
What is Acid Dissociation in Water?
With liquid water, dissolved hydroxide and solvated protons are in equilibrium. Typically, we use a value related to the equilibrium constant to define the concentration of solvated ions in water. The constant Kw for the dissociation of water is 1 x 10-14.
pH and pOH are important parameters for acidic and basic solutions. The log base 10 of the concentration of hydrogen ions or the log base 10 of the concentration of hydroxide ions, respectively.
- pH = -log [H+]
- pOH = -log [HO–]
- 14 = pH + pOH
According to the LeChateliers principle, introducing one of the products to a system in equilibrium shifts the equilibrium towards the reactants. Acids and bases dissolve in water and inhibit water dissociation by raising the concentration of either protons or hydroxide ions, the result of water’s self-ionization.
Typically, acidic and basic solutions in water are defined using pH and pOH. In pure water, the concentration of solvated protons is equal to that of solvated hydroxide anions, and the pH is 7. The pH of acidic solutions is less than that of basic solutions, which is greater.
Strong Acids and Water Dissociation
Strong acids dissociate completely in water and have negative Ka values. We can assume that the [H+] in a solution of a strong acid is equal to the original concentration of the acid. This section contains a list of strong acids.
AH + H2O → A– + H+

Weak Acids and Water Dissociation
For weak acids, there exists a condition in which the [H+] resulting from acid dissociation is comparable to the [H+] resulting from water dissociation (1.010-7 M). Water dissociation can contribute to the pH of a weak acid solution if the acid is extremely diluted, very weak, or both diluted and weak, however in the case of strong acids, it is only significant when the acid is extremely diluted.
In an aqueous solution, weak acids only partially dissociate. pKa = -log (Ka). The following table lists many weak acids:
| Acid | Reaction | pKa |
| Hydrofluoric acid | HF ⇌ H+ + F– | 3.17 |
| Carbonic acids | H2CO3 ⇌ H+ + HCO3– | 6.37 |
| Bicarbonate | HCO3– ⇌ H+ + CO3-2 | 10.25 |
| Bisulfate | HSO4– ⇌ H+ + SO4-2 | 1.99 |
| Ammonium | NH4+ ⇌ H+ + NH3 | 9.24 |
| Hydrogen sulfide | H2S ⇌ H+ + HS– | 7.0 |
| Water | H2O ⇌ H+ + HO– | 15.74 |
Acidity in nonaqueous solutions
A solvent is more likely to cause ionization of a dissolved acidic molecule under the following conditions:
- As a protic solvent, it is capable of forming hydrogen bonds.
- It has a big base of donors, showing that it is a strong Lewis base.
- Due to its high dielectric constant (relative permittivity), it is an outstanding solvent for ionic species.
DMSO and acetonitrile are widely employed to estimate the pKa values of organic compounds (ACN).
Acid ionization is less prevalent in an acidic solvent than in water. Hydrogen chloride becomes a mild acid when dissolved in acetic acid, for example. This is the case because acetic acid is a much weaker base than water.
HCl + CH3CO2H ⇌ Cl– + CH3C+(OH)2
acid + base ⇌ conjugate base + conjugate acid
Frequently Asked Questions – FAQs on Acid Dissociation
Q.1 When acids dissociate in water, what happens?
In an aqueous solution, an acid dissociates into hydrogen ions (H+) and anions. The molecules of a strong acid dissociate, resulting in a high concentration of H+.
Q.2 What is the definition of a protic solvent?
A protic solvent contains a hydrogen atom that is covalently bonded to oxygen (as in a hydroxyl group), nitrogen (as in an amine group), or fluoride (as in a fluoride group) (as in hydrogen fluoride).
Any solvent containing a labile H+ is known as a protic solvent. Typically, solvent molecules rapidly transfer protons (H+) to solutes via hydrogen bonding. Water is the most common protic solvent.
Q.3 basic terms, what is the equilibrium constant?
This number expresses the relationship between the number of products and reactants present at equilibrium in a reversible chemical reaction at a specific temperature.
Q.4 What is the definition of Lewis acid and Lewis base?
Lewis acids and bases are, respectively, electron-pair acceptors and donors. Consequently, a Lewis base can donate a pair of electrons to a Lewis acid, resulting in a coordinated covalent connection. The item is also known as a Lewis adduct.

