What is Acid Chloride
Acid Chlorides, also known as Acyl Chlorides, are a class of chemical compounds with a -COCl functional group. The oxygen atom is double-bonded to the carbon atom, whereas the chlorine atom is connected to the identical carbon atom by a single atom. The (C=O) group is known as the carbonyl group, but when combined with chlorine, it is known as acyl chloride.
Acid Chloride is a class of chemical compounds whose functional group is -COCl and whose overall formula is RCOCl.
Acid Chloride Questions with Solutions
Q1: Formyl chloride is:
- CHClO
- CH3Cl
- CH2OCl
- None of the above
Answer: (a)
Explanation: The formula of formyl chloride is CHClO.
Q2. Give a few examples of Acid Chlorides.
Answer: Some examples of Acid Chlorides are given below.
Ethanoyl chloride (CH3COCl), benzoyl chloride (C7H5ClO) and propanoyl Chloride (C2H5COCl).
Q3. Give an application of Acid Chlorides.
Answer: Acid Chlorides are used to prepare acid anhydrides, amides and esters. This can be done by bringing out the reaction of Acid Chlorides with a carboxylic acid salt, an amine and alcohol, respectively.
Q4. What is the hydrolysis product of Acid Chlorides?
Answer: The Acid Chlorides release the respective carboxylic acid on hydrolysis.
RCOCl + H2O → RCOOH + HCl
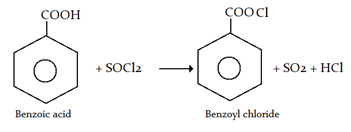
Q5. Benzoyl chloride can be prepared from benzoic acid by:
- Chlorine, h𝜈
- SO2, Chlorine
- SOCl2
- Chlorine, water
Answer: (c)
Explanation: On reaction with SOCl2, benzoic acid forms benzoyl chloride, along with the formation of SO2 and HCl.

Q6. What is meant by Acid Chlorides?
Answer: Acid Chlorides are a class of organic compounds that are formed when the hydroxyl group (-OH) of the (-COOH) group of the carboxylic acid is replaced by a chlorine atom. However, the acid can be restored by the hydrolysis of the Acid Chloride.
Q7. What is the reason behind the high reactivity of the Acid Chlorides?
Answer: Acid Chlorides are the most reactive derivatives of the carboxylic acid functional groups. This is because the chlorine atom attached to the CO group is highly electronegative and pulls the shared pair of electrons in the C-Cl bond towards itself. This makes the C=O carbon even more electrophilic. As a result, the ease of nucleophilic attack increases on the carbonyl carbon. The chlorine atom is a good leaving group, so it leaves quickly as the reaction takes place.
Q8. The reaction of formic acid with PCl5 forms:
- Acetyl chloride
- Formyl chloride
- Methyl chloride
- Propanoyl chloride
Answer: (b)
Explanation: Formyl chloride is formed by the reaction of formic acid and PCl5.
HCOOH + PCl5 → HCOCl + POCl3 + HCl
Q9. Acetyl chloride can be prepared in the laboratory by:
- The reaction of acetic acid with thionyl chloride (SOCl2)
- The reaction of acetic acid with phosphorus trichloride (PCl3)
- The reaction of sodium acetate with phosphorus trichloride (PCl3)
- None of the above
Answer: (a)
Explanation: Acetyl chloride is formed by the reaction of acetic acid (CH3COOH) and thionyl chloride (SOCl2).
CH3COOH + SOCl2 → CH3COCl + SO2 + HCl
Q10. Benzyl chloride on _____ gives benzoic acid.
Answer: Oxidation
Explanation: Benzoic acid is formed by the oxidation of benzyl chloride.

Q11. Why are Acid Chlorides acidic in nature?
Answer: In the anhydrous form, the Acid Chlorides remain neutral due to the unavailability of the H+ ions. In an aqueous solution, the Acid Chlorides dissociate to form carboxylic acid and HCl. Now that HCl is a strong acid, it dissociates completely in the solution to give H+ ions. These H+ ions are the characteristic of the acidity of the solution. Thus, Acid Chlorides have an acidic nature.
Q12. What is ‘D’ in the following reaction?
C6H5CCl3 + C6H5CO2H → 2C6H5COCl + D
- Chlorine (Cl2)
- Water (H2O)
- Hydrochloric acid (HCl)
- Hydrogen gas (H2)
Answer: (c)
Explanation: The reaction of benzotrichloride (C6H5CCl3), either with water (H2O) or with benzoic acid (C6H5CO2H), forms benzoyl chloride (C6H5COCl) and hydrochloric acid (HCl).
Q13. How are the Acid Chlorides named?
Answer: The basic rule of naming the Acid Chlorides is just like naming any other organic compound. The longest carbon chain is selected and numbered beginning from the Acid Chloride group carbon. To the parent carbon chain name, the suffix ‘oyl chloride’ is added.
For example, CH3CH2COCl is named Propanoyl chloride.
Q14. What Acid Chlorides form on reacting with the carboxylic acids?
Answer: The Acid Chlorides on reaction with carboxylic acids form acid anhydrides.
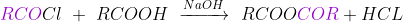
Q15. What is ‘A’ in the following reaction?
2C6H5COCl + H2O2 + 2NaOH → A + 2NaCl + 2H2O
- Chlorophenol (C6H5ClO)
- Chlorotoluene (C7H7Cl)
- Chlorobenzene (C6H5Cl)
- Benzoyl Peroxide (C14H10O4)
Answer: (d) Benzoyl Peroxide (C14H10O4)
Explanation: The reaction of benzoyl chloride with hydrogen peroxide and sodium hydroxide is the industrial preparation method for Benzoyl Peroxide. The complete reaction for the same is as follows:
2C6H5COCl + H2O2 + 2NaOH → (C6H5CO)2O2 + 2NaCl + 2H2O
Q16. Benzoyl chloride is prepared from benzoic acid by which of the following?
a) Cl2, hv
b) SO2, Cl2
c) SOCl2
d) Cl2, H2O
Answer: c) SOCl2
Explanation: Benzoyl chloride is prepared from benzoic acid be SOCl2.