What is Acetone?
Acetone is an organic compound with the formula (CH3COCH3). It is the most elementary and smallest ketone. It is an odorless, extremely volatile, and combustible liquid with a distinctive pungent odor. It is sometimes known as propanone. It is present in the combustion of automobiles, plants, and forest fires. It is also typically present in urine and blood in the human body.
The formula for acetone (or propanone) is provided in both organic and structural forms. Read the linked article to learn more about acetone, its structure, and its properties.
Acetone Formula
The structural formula and organic formula of acetone are listed here, along with its structure. The structure will facilitate a better understanding of this molecule and the organic formula.
Structural Formula of Acetone (Propanone)
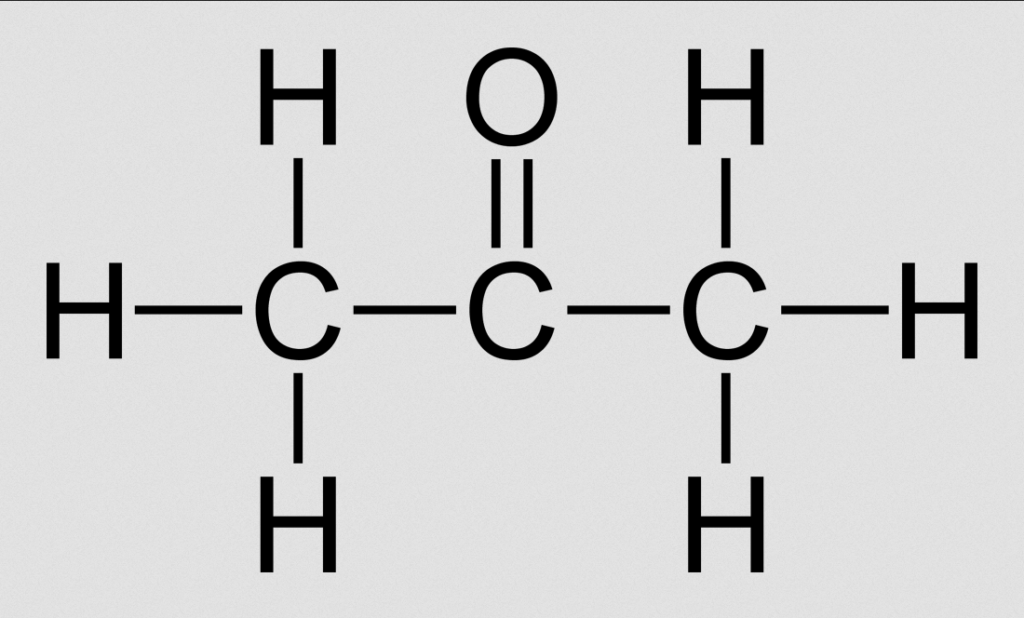
Organic Formula (Chemical Formula) of Acetone
Three carbon atoms, six hydrogen atoms, and one oxygen atom make up acetone. It is regarded to be a ketone since it contains a carbonyl group. Consequently, the chemical formula for acetone or propanone is:
Acetone Chemical Formula = C3H6O
Properties of Acetone
| Acetone | C3H6O |
| Appearance | Colorless liquid |
| Odor | Pungent, irritating, floral |
| Boiling Point | 56oC |
| Melting Point | -95oC |
| Molecular mass | 58.08 g/mol |
| Density | 0.7845 g/cm3 (25 °C) |
Uses of Acetone
Acetone is mostly used in medicine and cosmetics. Acetone is found in both blood and urine. In addition to being an active ingredient in nail polish removers, acetone is also a component of nail polish removers.
Visit the uses of acetone to discover more about its applications in various industries.
Acetone is applied in various fields. Several of these are detailed below.
- Medicine or pharmaceutical industry
- Used in cosmetics
- Used in laboratory
- Used in electronics
- Used for domestic purposes
Frequently Asked Questions – FAQs
Q.1 What acetone is used for?
Acetone is a chemical used to create nail polish removers and paint thinners. This molecule is also produced when your body breaks down fat.
Q.2 Is acetone harmful to humans?
A moderate amount of acetone inhaled over a brief period of time can irritate the nose, throat, lungs, and eyes. A woman’s menstrual cycle can be shortened by headache, vertigo, confusion, tachycardia, nausea, vomiting, bleeding, the chance of fainting, and coma.
Q.3 Is acetone heavier than water?
At normal temperatures, acetone exists as a liquid, but its density is less than around 1 g/mL of water. The density of acetone at normal temperature is 0.788g/mL. This indicates that the liquid has a mass of 0.7888 grams per milliliter.
Q.4 Why is acetone water-soluble?
Polar solutes dissolve in polar solvents while nonpolar solutes dissolve in nonpolar solvents, according to the “like dissolves like” concept. Due to the polar nature of both acetone and water, it is soluble in water. Due to the establishment of intermolecular hydrogen bonds between the polar carbonyl group and water molecules, acetone is also soluble in water.
Q.5 Is acetone better than water as a solvent?
Because of its capacity to dissolve both polar and nonpolar chemicals, acetone is a good solvent. However, other solvents can also dissolve either type of substance. Second, acetone is an effective solvent because it is miscible. That is, it is capable of combining with water in any amount.

