What is an Amide?
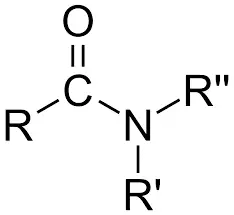
Amides are compounds with a carbonyl functional group connected to an amine and a hydrocarbon group (or hydrogen atom). A carbonyl functional group consists of a carbon atom with double bonds and an oxygen atom.
R(C = O)NR’R”, where R, R’, and R” denote a hydrocarbon or hydrogen substituent, is the general structure of an amide.
You can Read More Chemistry Articles.
What is an Amido?
The distinction between Amido and Amides is minimal. Amido implies the presence of an amide group in chemistry.
Synthesis of Amides
Amine Acylation
An amide is produced when an amine reacts with an acyl group (a carboxylic acid derivative).
In amines, the nitrogen atom possesses a pair of unpaired electrons that attack the electron-deficient carbonyl carbon and establish a covalent connection with it. The equivalent amide is produced by the removal of a proton (from the amine substituent) and the leaving group.
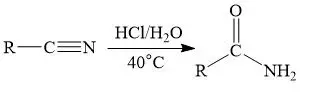
Nitrile Hydrolysis
Under acidic or basic circumstances, partial hydrolysis of nitriles (RCN) results in the creation of amides.
Beckmann Rearrangement
Beckmann rearrangement is among the most essential amide synthesis techniques.
This reaction produces an amide from an oxime group. The synthesis of an amide arises from the rearrangement of oximes in the presence of an activating agent, such as H2SO4 or PCl3.
Properties of Amides
- An amide is made when the nitrogen atom of an amine is joined to a carboxyl group.
- The polarity of the amide bond allows it to create hydrogen bonds with water. Consequently, amides are soluble in water (but only in the lower members). As the molar mass of an amide’s hydrocarbon substituent grows, its solubility drops.
- Due to the amide’s solubility, its boiling and melting points are high.
- Amides are less basic than amines because the lone pair of electrons on the nitrogen atom of the amide is conjugated with the carbonyl group, so reducing the availability of electrons for donation.
Examples of Amides
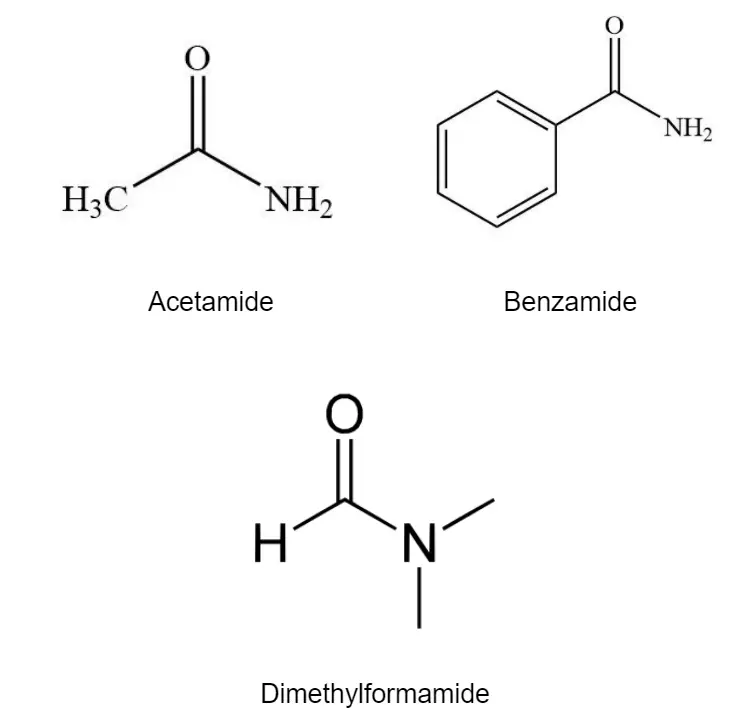
Acetamide H3C-CONH2, benzamide C6H5CONH2, and dimethylformamide HCON(CH3)2 are examples of amides.
Classification of Amides
On the basis of the substituents connected to the nitrogen atom of an amide, three distinct types of amides exist.
There are three categories of amides: primary, secondary, and tertiary.
- A fundamental (1°) amide contains solely hydrogen atoms as substituents on the nitrogen atom of the amide.
- The nitrogen atom of a secondary (2°) amide is connected to a hydrocarbon substituent.
- In a tertiary (3°) amide, the nitrogen is linked to three carbons.
- The term lactam refers to a cyclic amide.

Applications
Amides are commonly employed as structural materials in technology. The formation of an amide bond is straightforward, it is resistant to hydrolysis, and it gives structural stiffness. The most resilient substances are polyamides and nylons. A number of medications, including penicillin, LSD, and paracetamol, are amides. In addition, plant N-alkylamides provide an array of biological activities.
Frequently Asked Questions on Amido and Amide
Q.1 What is the distinction between an amide and an amine?
The existence of a carbonyl group in their structures is the major contrast between amine and amide; amines have no carbonyl groups connected to the nitrogen atom, whereas amides contain a carbonyl group bonded to the nitrogen atom.
Q.2 What are the examples of amides?
The amides Nylon, Paracetamol, and Dimethylformamide are examples. Ammonia-derived amides are the most fundamental amides. Amphetamines, in general, are fairly weak bases.
Q.3 What are the three different types of amides?
On the basis of their names, amides are divided into three distinct categories: primary amide, secondary amide, and tertiary amide.
Q.4 What are the properties of amides?
Generally, the boiling and melting points of amides are high. These qualities, as well as their solubility in water, result from the polarity and hydrogen bonding of the amide group.
Q.5 How are amides synthesised?
An acid anhydride can be used to create simple amides by reacting with an amine. amides can also result from a direct reaction between a carboxylic acid and an amine.

