What is Alkyl Iodide
Due to the alkyl iodides‘ lower stability compared to fluorides, chlorides, or bromides as well as the fact that it is somewhat more expensive, other halogens are now used to create alkyl halides. These alkyl iodides function as synthetic intermediates and, as a result, have distinct advantages over alkyl bromides, making them a less common ingredient in the production of alkyl halides.
You can Read More Chemistry Articles.
Observing that alkyl iodide hydrolyses faster, it can be concluded that the strength of the C-X bond has a greater effect on the rate than the degree of polarisation of the bond. Additionally, we know that compounds with weaker bonds hydrolyze more rapidly. The rate is proportional to the difference in energy between the starting material and the product. This is because the activation energy is typically lower.
As alkyl iodide is less stable than alkyl chloride because the C-X bond is weaker. In addition, the C-X bond of alkyl chloride is more polarized than the C-I bond of alkyl iodide, and the C-Cl bond has greater electronegative bond energy than the C-I bond. There are a great deal of fascinating facts about these halides.
Alkyl Iodide Synthesis
The photochemical iodination of alkanes with elemental iodine is well-known, but it is of little or no importance. iodination of carbonyl compounds and their enol derivatives is significantly easier to achieve than this. For activated methylene groups such as malonates, the iodination process is straightforward using K2CO3 as the base and I2 as one of the halogen sources in phase transfer catalysis.
- Alkyl halides can also be produced by the addition of iodine to alkenes. This is possible by adding elemental iodine across double bonds to produce vicinal di-iodo compounds, but it is of limited utility for synthesis because the reverse reaction is thermodynamically preferred.
- Alkyl iodides are the least stable of the alkyl halides, but they can be prepared easily via SN2 halide exchange under the classical condition of Finkelstein. Although halide exchange is the reversible treatment of an alkyl bromide or chloride with a sodium iodide solution in acetone at reflux, alkyl iodide is produced. This is because the precipitation of sodium chloride, which is less soluble in acetone than sodium iodide, shifts the equilibrium positions.
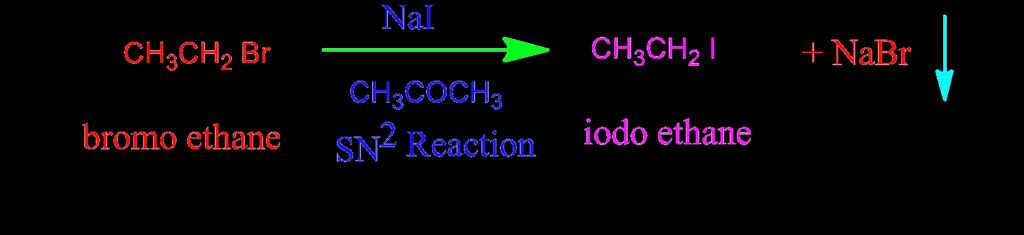
- Due to the SN2 nature of halide substitution, secondary and tertiary halides are slow to react with iodide ions and typically require zinc or iron halide catalysis. Alkyl fluorides, alkyl bromides, and alkyl chlorides can be converted into iodides by heating with excess aqueous HI, with or without phase transfer catalysis.
- For the conversion of alkyl bromides to iodides, the generally poor solubility of sodium or potassium iodides has been overcome through the use of dipolar aprotic solvents, such as the addition of crown ether to solubilize the metal counterion, and phase transfer catalysis.
- By reacting with trimethylsilyl iodide, tertiary alkyl nitro compounds are transformed into corresponding iodides. Because primary and secondary nitroalkanes produce nitriles and oximes, this reaction is restricted to tertiary systems only.
Alkyl Iodide Reactions
Alkyl iodides undergo elimination reactions with nucleophiles or bases, resulting in the loss of hydrogen iodide and the formation of an alkene. E1 and E2 are two of the most prevalent mechanisms.
- The E2 mechanism is the most preferred and efficient method for synthesizing alkenes from alkyl iodides, and it can be applied to the synthesis of primary, secondary, and tertiary alkyl iodides.
- The E1 reaction is not especially useful from a synthetic standpoint and occurs simultaneously with the SN1 reaction. Using this mechanism, tertiary alkyl iodide and some secondary alkyl iodides can react.
- In such cases, the primary forms are not formed. The E2 mechanism is a single-step process involving both alkyl iodide and nucleophile; the reaction is classified as second-order and depends on the concentrations of both reactants.
- The E1 mechanism is a two-step process involving the loss of the halide to form a carbocation and then the loss of a susceptible proton to form an alkene. The rate-determining step is the initial stage involving halide ion loss, so the reaction is considered to be of the first order.
- The intermediate carbocation is stabilized by substituent alkyl groups.
- In monomolecular substitution SN1, the C-X bond in alkyl halide is first broken, followed by the formation of a carbonium ion. Then, a rapid reaction with the nucleophilic agent occurs.
- In contrast to the other reaction, the SN2 reaction is second-order in terms of both the alkyl halide and the nucleophilic agent. Only with respect to an alkyl halide is the SN1 reaction of first-order.
So we can also say that in the first case, the rate of the reaction is proportional to the amount of both reacting species, and in the second case, the rate of the reaction depends only on the amount of alkyl halide, or in this case, alkyl iodide.
In less polar solvents, the reaction rate of thiourea with alkyl iodide decreases dramatically as follows.
Initial > Secondary > Third
Increased medium polarity facilitates the SN1 reaction mechanism, making tertiary alkyl halide more reactive.
RX → R+ + X–
The branching of the carbon chain, the possible presence of a double bond, and the location of a second halogen atom also affect the reactivity. The most reactive monovalent and divalent halo compounds tested are methyl iodide and methylene iodide, as well as benzyl bromide and allyl bromide.
Why Can’t Alkyl Iodides be Prepared Directly?
- Alkyl lithium compounds are typically produced by reacting lithium metal with an alkyl halide in the presence of a hydrocarbon or ethereal solvent and dry nitrogen or argon.
- Alkyl chlorides are preferred over alkyl iodide because these compounds react with alkyl lithium very rapidly. Using double titration, the concentrations of n-butyl lithium solutions can be determined.
- Diethyl ether is attacked by lithium alkyl, which is less stable in tetrahydrofuran. If iodine and red mercuric oxide are used, acids can also form alkyl iodides. It is possible to convert aliphatic carboxylic acids into esters and alkyl halides:
- By reacting lead tetraacetate and lithium chloride with carboxylic acid in benzene, alkyl chloride can be produced without simultaneous ester formation. Again, the iodide forms of alkyls cannot be considered because these forms react rapidly and are therefore undesirable.
Alkyl Iodide Free Radical Halogenation
Iodine can be used in the free radical mechanism with the aid of activating light with a wavelength of 184.9 nm. However, it is important to note that iodination using I2 alone is rarely used, and if it is, it may be because the formed hydrogen iodide reduces the alkyl iodide.
Compared to other forms of halogens, the direct free radical halogenation method of aliphatic hydrocarbons using iodine is significantly endothermic in nature. Under such conditions, the required chain reaction cannot occur.
Iodo alkane is produced when iodine from aluminum iodide (AlI3) reacts with an alkane in dibromo methane at 20 degrees Celsius. Alcohols derived from alkanes can be utilized in a nucleophilic substitution reaction to produce alkyl halides. This is accomplished through the halogenation of free radicals and the addition of hydrogen halide from alkenes. These molecules are considered precursors for the synthesis of other functionally substituted organic compounds through the replacement of halide with a nucleophile.
Alkanes are unreactive because they are non-polar and lack functional groups that can undergo reactions. Therefore, alkanes can be functionalized through the process of free radical halogenation.
Limitations of Radical Halogenation
The number of similar C-H bonds present in all but the simplest alkanes is a limitation of radical halogenation, making reactions difficult to achieve. There are three stages involved in these reactions.
- The initiation steps, followed by the propagation steps, are followed by the termination steps.
- During the step of propagation, a halogen radical abstracts a hydrogen from methane to produce a methyl radical.
- Following this is the regeneration of the halogen atom.
- The halogen molecule is regenerated when the halogen atom reacts with another chlorine atom. The combination of two methyl radicals produces ethane.
- As the reaction progresses, the lack of selectivity in the halogenation of methane becomes evident.
- The concentration of halogen (iodo) methane increases as it is created. As the methyl halogen has C-H bonds, it can also undergo iodination to form di-iodo methane or dichloromethane, which is the preferred product.
Frequently Asked Questions – FAQs
Q.1 Why are alkyl halides naturally reactive?
Due to the significant difference in electronegativity between carbon and halogen atoms, the bond between carbon and hydrogen in alkyl halide is highly polarized. So that they can easily separate into various products.
Q.2 Which alkyl halide is more reactive?
The order of alkyl halide reactivity is RI > RBr > RCl > RF. Alkyl iodide is more reactive because the bond between carbon and iodine is formed through the overlap of 2p and 5p orbitals, which are easily broken.
Q.3 Why do alkyl iodides become violet or brown upon standing?
The bond dissociation energy of alkyl iodide is extremely low, so it readily dissociates in the presence of air and sunlight to form violet or brown iodide ion.
Q.4 How do you convert alcohol to alkyl iodide?
Alcohols derived from alkanes can be utilized in a nucleophilic substitution reaction to produce alkyl halides. This is accomplished through the halogenation of free radicals and the addition of hydrogen halide from alkenes.
Q.5 How do you convert an alkyl chloride to alkyl iodide?
Alkyl iodides are the least stable of the alkyl halides, but they can be prepared easily via SN2 halide exchange under the classical condition of Finkelstein. Alkyl chloride reacts with a solution of sodium iodide in the presence of acetone derived from alkyl iodide in this reaction.

