Class 12 Chemistry CBSE Lab Manual 2.5
CBSE Class 12 Chemistry Lab Manual 2.5 – To Prepare aluminium hydroxide, [Al(OH)3] sol
Chemistry Practicals Class 12 CBSE Lab Manual – Free PDF Download
Class 12 Chemistry CBSE Lab Manual Chapters wise Download here in pdf format. These research lab Manual is also freely downloadable and used as a book of facts. Learning doesn’t mean solely gaining information regarding facts and principles rather it’s a path that is faithful by scientific truths, verified by experimentation. Keeping these facts in mind, CBSE research lab manual for Class 12 are planned, evaluated below subject Improvement Activities. Check our CBSE research lab manual for Class 12. we tend to square measure grateful to the academics for his or her constant support provided within the preparation of this CBSE research lab Manual.
Study Without Internet – Offline Download Now
Class 12 Lab Manual Chemistry Practical 2.5 – To Prepare aluminium hydroxide

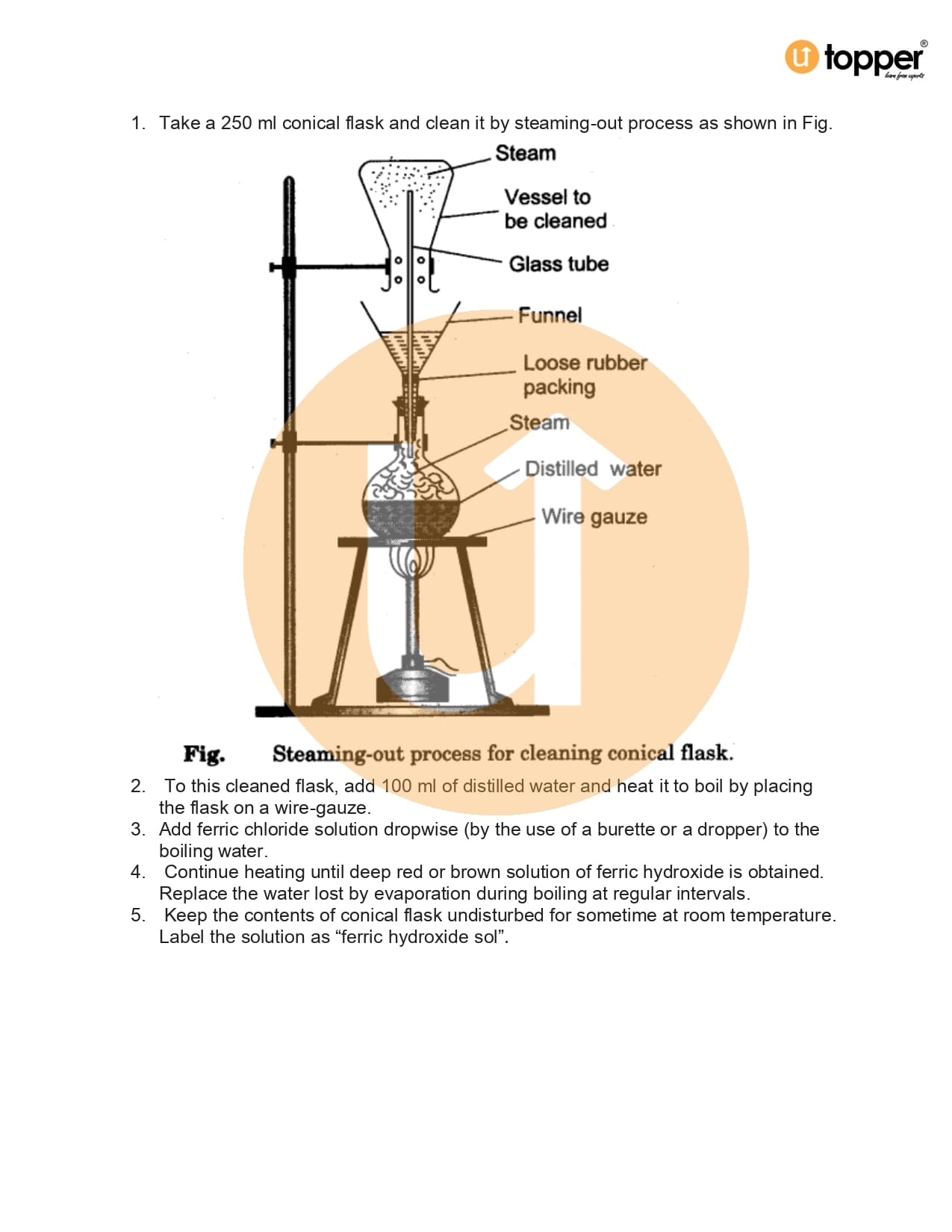
Class 12 Chemistry Practicals CBSE Lab Manual Download PDf
- Introduction to Basic Laboratory Equipment
- Surface Chemistry
- Exp-2.1 : To prepare colloidal solution (sol) of starch.
- Exp-2.2 : To prepare a colloidal solution of gum.
- Exp-2.3 : To prepare colloidal solution (or sol) of egg albumin.
- Exp-2.4 : To prepare ferric hydroxide, [Fe(OH)3] sol.
- Exp-2.5 : To prepare aluminium hydroxide, [Al(OH)3] sol.
- Exp-2.6 : To prepare colloidal solution of arsenious sulphide, [As2 S3].
- Exp-2.7 :To study the dialysis of starch sol containing sodium chloride through a cellophane or parchment paper.
- Exp-2.8 : Compare the precipitation values of sodium chloride, barium chloride and aluminium chloride for arsenious sulphide sol.
- Exp-2.9 : To study the effectiveness of different common oils (castor oil, cotton seed oil, coconut oil, kerosene oil, mustard oil) in forming emulsions.
- Exp-2.10 : To compare the effectiveness of a number of emulsifying agents in forming emulsions.
- Surface Chemistry Viva Questions with Answers.
- Chemical Kinetics
- Exp-3.1 : To study the effect of concentration on the rate of reaction between sodium thiosulphate and hydrochloric acid.
- Exp-3.2 : To study the effect of change in temperature on the rate of reaction between sodium thiosulphate and hydrochloric acid.
- Exp-3.3 : To study the reaction rate of reaction of iodide ions with hydrogen peroxide at different concentrations of iodide ions.
- Exp-3.4 : To study the reaction rate of the reaction between potassium iodate (KIO3) and sodium sulphite (Na2S03) using starch solution as indicator.
- Chemical Kinetics Viva Questions with Answers.
- Thermochemistry
- Exp-4.1 : Determine the calorimeter constant (W) of calorimeter (polythene bottle).
- Exp-4.2 : Determine the enthalpy of dissolution of given solid copper sulphate (CuS04.5H20) in water at room temperature.
- Exp-4.3 : Determine the enthalpy of neutralisation of hydrochloric acid with sodium hydroxide solution.
- Exp-4.4 : Determine the enthalpy change during the interaction (hydrogen bond formation) between acetone and chloroform.
- Thermochemistry Viva Questions with Answers.
- Electrochemistry
- Exp-5.1 : To set up simple Daniell cell and determine its emf .
- Exp-5.2 : To set up simple Daniell cell using salt bridge and determine its emf .
- Exp-5.3 : To study the variation of cell potential in Zn | Zn2+ || Cu2+ | Cu cell with change in concentration of electrolytes (CuS04 and ZnS04) at room temperature.
- Electrochemistry Viva Questions with Answers.
- Chromatography
- Exp-6.1 : Separate the coloured components present in the mixture of red and blue inks by ascending paper chromatography and find their Rf values .
- Exp-6.2 : Separate the coloured components present in the given grass/flower by ascending paper chromatography and determine their Rf values .
- Exp-6.3 : Separate Co2+ and Ni2+ ions present in the given mixture by using ascending paper chromatography and determine their Rf values .
- Chromatography Viva Questions with Answers.
- Preparation of Inorganic Compounds
- Exp-7.1 : To prepare a pure sample of ferrous ammonium sulphate (Mohr’s salt), [FeSO4 . (NH4)2 SO4.6HO20] .
- Exp-7.2 : To prepare a pure sample of potash alum (Fitkari), [K2SO4.Al2 (SO4)3. 24H20] .
- Exp-7.3 : To prepare a pure sample of the complex potassium trioxalatoferrate(III), Kg[Fe(C2O4)3l . 3H20 .
- Preparation of Inorganic Compounds Viva Questions with Answers.
- Preparation of Organic Compounds
- Exp-8.1 : To prepare a sample of acetanilide from aniline.
- Exp-8.2 : To prepare a sample of dibenzalacetone.
- Exp-8.3 : To prepare a sample of p-nitroacetanilide from acetanilide .
- Exp-8.4 : To prepare 2-naphthol aniline or phenyl-azo-β-naphtholdye .
- Preparation of Organic Compounds Viva Questions with Answers.
- Tests for the Functional Groups Present in Organic Compounds
- Tests of Carbohydrates, Fats and Proteins in Pure Samples and Detection of Their Presence in Given Food Stuffs
- Exp-10.1 : To study some simple tests of carbohydrates
- Exp-10.2 : To study some simple tests of oils and fats .
- Exp-10.3 : To study some simple tests of proteins .
- Exp-10.4 : To detect the presence of carbohydrates, fats and proteins in the following food stuffs : Grapes, potatoes, rice, butter, biscuits, milk, groundnut, boiled egg .
- Tests of Carbohydrates, Fats and Proteins in Pure Samples and Detection of Their Presence in Given Food Stuffs Viva Questions with Answers.
- Volumetric Analysis
- Exp-11.1 : Prepare 250 ml of M/10 solution of oxalic acid from crystalline oxalic acid .
- Exp-11.2 : Prepare 250 ml of a N/10 solution of oxalic acid from crystalline oxalic acid .
- Exp-11.3 : Preparation of 250 ml of M/20 solution of Mohr’s salt .
- Exp-11.4 : Preparation of 250 ml of N/20 solution of Mohr’s salt .
- Exp-11.5 : Prepare M/20 solution of ferrous ammonium sulphate (Mohr’s salt). Using this solution find out the molarity and strength of the given KMn04 solution.
- Exp-11.6 : Prepare a solution of ferrous ammonium sulphate (Mohr’s salt) containing exactly 17.0 g of the salt in one litre. With the help of this solution, determine the molarity and the concentration of KMnO4 in the given solution.
- Exp-11.7 : Prepare M/20 ferrous ammonium sulphate (Mohr’s salt) solution. Find out the percentage purity of impure KMnO4 sample 2.0 g of which have been dissolved per litre .
- Exp-11.8 : Determine the equivalent mass and number of molecules of water of crystallisation in a sample of Mohr’s salt, FeSO4(NH4)2 SO4 . nH20. Provided KMnO4.
- Exp-11.9 : Prepare M/50 solution of oxalic acid. With its help, determine 50 the molarity and strength of the given solution of potassium permanganate (KMnO4).
- Exp-11.10 : Find out the percentage purity of impure sample of oxalic acid. You are supplied M/100 KMnO4 solution.
- Exp-11.11 : The given solution has been prepared by dissolving 1.6 g of an alkali metal permanganate per litre of solution. Determine volumetrically the atomic mass of the alkali metal. Prepare M/20 Mohr’s salt solution for titration.
- Exp-11.12 : Determine the percentage composition of a mixture of sodium oxalate and oxalic acid . Provided M/100 KMnO4 solution.
- Exp-11.13 : You are provided with a partially oxidised sample of ferrous sulphate (FeSO4.7H20) crystals. Prepare a solution by dissolving 14.0 g of these crystals per litre and determine the percentage oxidation of the given sample. Given M/100 KMnO4 solution.
- Exp-11.14 : Calculate the percentage of Fe2+ ions in a sample of ferrous sulphate. Prepare a solution of the given sample having strength exactly equal to 14.0 g/litre. Provided M/100 KMnO4 .
- Exp-11.15 : Prepare N/20 Mohr’s salt solution. Using this solution, determine the normality and strength of the given potassium permanganate solution.
- Exp-11.16 : Prepare N/20 solution of oxalic acid. Using this solution, find out strength and normality of the given potassium permanganate solution .
- Exp-11.17 : Determine the percentage purity of the given sample of oxalic acid. Ask for your requirement .
- Exp-11.18 : Determine the percentage composition of a mixture of sodium oxalate and oxalic acid Provided N/20 KMnO4 .
- Exp-11.19 : Determine the equivalent mass and number of molecules of water of crystallisation in a sample of Mohr’s salt FeSO4 (NH4)2 SO4.nH20. Provided N/20 KMnO4.
- Volumetric Analysis Viva Questions with Answers.
- Chemistry Qualitative Analysis
CBSE lab Manual for Class 12 Features:
- Basic conception of Experiments
- Before playacting the experiments the fundamental conception section of each experiment helps the scholars in recognize the aim of the experiment and to realize the result with the minimum mistake
- Lab Experiments with Interactive session and NCERT research lab Manual queries
- Completely solved CBSE research lab Manual queries square measure provided.
- Practical primarily based queries
By playacting the experiments, students can recognize the conception in an exceedingly higher method as they’ll currently read the changes happening ahead of their eyes. Their basics can become solid as they’re going to learn by doing things. By doing this activity they’re going to conjointly get generated their interest within the subject. Students can develop questioning skills and begin learning from a scientific perspective. Here we’ve got given all the required details that a Class 9 student ought to realize CBSE research lab Manual. From CBSE Science sensible to research lab manual, project work, vital queries and CBSE research lab kit manual, all the data is given within the elaborate kind additional during this page for Class 9 students.

Preparation Tips
For clearing Board Exams for the students. they’re going to need to possess a well-structured commit to study. The communicating are conducted within the month of could per annum. Students got to be sturdy academically in conjunction with numerous different skills like time management, exam-taking strategy, situational intelligence and analytical skills. Students got to harden.
this examination well-in advanced.
- Time-management
- Focus on vital topics
- Plan your day ahead
- The apply is that the key to success
- Take tests when each thought
- Regular revision
FAQ ( Frequently Asked Questions )
1. Can I Download the Class 1 Ncert Books PDF ?
Ans – Yes , You can Download the Ncert Books Directly From the Website of Utopper
2. How To Download the Ncert Books Pdf ?
Ans – Please click the Class of which you want to Download the Ncert Books and there is direct pdf Links Given on Our Website
3. Is Ncert Books Difficult ?
Ans – NCERT Books are designed in such a way that it covers the basic knowledge of your syllabus. It’s every line consist of deep and useful meaning. If you complete ncert books it would be very helpful many other extra refernce books have taken their bases and references from ncert .
4. Why NCERT Books are Important ?
Ans – NCERT Books Strictly follows the CBSE curriculum and it Clears all the fundamental concepts of students by offering a number of problems to practice and it is BEST to score Good Marks in CBSE Board Exam.


